Citrullinated Autoantigen-Specific T and B Lymphocytes in Rheumatoid Arthritis: Focus on Follicular T Helper Cells and Expansion by Coculture
- PMID: 39846262
- PMCID: PMC11755120
- DOI: 10.1002/acr2.11785
Citrullinated Autoantigen-Specific T and B Lymphocytes in Rheumatoid Arthritis: Focus on Follicular T Helper Cells and Expansion by Coculture
Abstract
Objective: Rheumatoid arthritis (RA) is characterized by circulating anti-cyclic citrullinated peptide (CCP) autoantibodies (ACPAs), resulting in inflammation of the joints and other organs. We have established novel assays to assess immune cell subpopulations, including citrullinated antigen-specific (CAS) autoreactive B and T lymphocytes, in patients with RA.
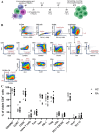
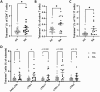
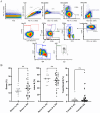
Methods and results: We found that activated CD25+ T cells were markedly increased in patients with RA compared to healthy controls. Novel combinations of major histocompatibility complex class II citrulline epitope tetramers were developed, which enabled robust detection of CAS T cells and showed increases of CAS-naive T helper cells, Th1.17 cells, CAS total circulating T follicular helper (cTfh) cells, and cTfh1 cells in ACPA+ patients with RA. In addition, an innovative assay using dual labeling with CCP-biotin probes allowed for reproducible identification of primary CAS B cells after enrichment with advantages over existing detection methods. Furthermore, patient-derived immune cells were successfully expanded. Primary RA B cells were successfully cultured on novel feeder cell lines, whereas T cells were expanded ex vivo in the presence of interleukin-2 and citrullinated peptides, and subsequent alterations in cell frequencies were assessed.
Conclusion: Novel assays were established to reliably detect CAS T and B cells in patients with RA, and specific CAS-naive T helper cells, Th1.17 cells, cTfh cells, and cTfh1 cells were observed more frequently in RA. Based on these results, new coculture systems of disease-relevant cells are developed to simulate human secondary lymphoid tissues ex vivo. This technology will serve as a platform to identify therapies that modulate disease-specific immune cells.
© 2025 The Author(s). ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures



References
-
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365(23):2205–2219. - PubMed
-
- Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 2005;4(3):130–136. - PubMed
-
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388(10055):2023–2038. - PubMed
-
- Albrecht K, Müller‐Ladner U. Side effects and management of side effects of methotrexate in rheumatoid arthritis. Clin Exp Rheumatol 2010;28(5 suppl 61):S95–S101. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

