Salt-Compact Albumin as a New Pure Protein-based Biomaterials: From Design to In Vivo Studies
- PMID: 39846332
- PMCID: PMC11912121
- DOI: 10.1002/adhm.202403385
Salt-Compact Albumin as a New Pure Protein-based Biomaterials: From Design to In Vivo Studies
Abstract
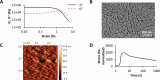
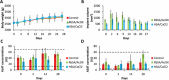
Current biodegradable materials are facing many challenges when used for the design of implantable devices because of shortcomings such as toxicity of crosslinking agents and degradation derivatives, limited cell adhesion, and limited immunological compatibility. Here, a class of materials built entirely of stable protein is designed using a simple protocol based on salt-assisted compaction of albumin, breaking with current crosslinking strategies. Salt-assisted compaction is based on the assembly of albumin in the presence of high concentrations of specific salts such as sodium bromide. This process leads, surprisingly, to water-insoluble handable materials with high preservation of their native protein structures and Young's modulus close to that of cartilage (0.86 MPa). Furthermore, these materials are non-cytotoxic, non-inflammatory, and in vivo implantations (using models of mice and rabbits) demonstrate a very slow degradation rate of the material with excellent biocompatibility and absence of systemic inflammation and implant failure. Therefore, these materials constitute promising candidates for the design of biodegradable scaffolds and drug delivery systems as an alternative to conventional synthetic degradable polyester materials.
Keywords: albumin; biodegradable materials; protein‐based materials; salt‐assisted compaction.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- de Jong H. G. B., Kruyt Koazervation H. R., Kolloid Z., 1930, 50, 39.
-
- Decher G., Eckle M., Schmitt J., Struth B., Curr. Opin. Colloid. In. 1998, 3, 32.
-
- Reisch A., Tirado P., Roger E., Boulmedais F., Collin D., Voegel J.‐C., Frisch B., Schaaf P., Schlenoff J. B., Adv. Funct. Mater. 2013, 23, 673.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

