Long-term safety and efficacy of ritlecitinib in adults and adolescents with alopecia areata and at least 25% scalp hair loss: Results from the ALLEGRO-LT phase 3, open-label study
- PMID: 39846397
- PMCID: PMC12105460
- DOI: 10.1111/jdv.20526
Long-term safety and efficacy of ritlecitinib in adults and adolescents with alopecia areata and at least 25% scalp hair loss: Results from the ALLEGRO-LT phase 3, open-label study
Erratum in
-
Correction to 'Long-term safety and efficacy of ritlecitinib in adults and adolescents with alopecia areata and at least 25% scalp hair loss: Results from the ALLEGRO-LT phase 3, open-label study'.J Eur Acad Dermatol Venereol. 2025 Sep;39(9):1687-1689. doi: 10.1111/jdv.20829. Epub 2025 Jul 12. J Eur Acad Dermatol Venereol. 2025. PMID: 40650473 Free PMC article. No abstract available.
Abstract
Background: ALLEGRO-LT is an ongoing, long-term, open-label, multicentre, phase 3 study of ritlecitinib in adults and adolescents with alopecia areata (AA).
Objectives: To evaluate ritlecitinib safety and efficacy through Month 24 in patients with AA and ≥25% scalp hair loss.
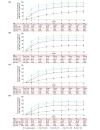
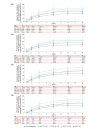
Methods: ALLEGRO-LT enrolled rollover patients who previously received study intervention in either ALLEGRO phase 2a or 2b/3 studies and de novo patients who had not received treatment in either study. The de novo cohort results are reported here. Patients aged ≥12 years with AA and ≥25% scalp hair loss received a daily, 4-week 200-mg ritlecitinib loading dose, followed by daily 50-mg ritlecitinib. Analyses are based on data up to the cut-off (December 2022). Efficacy outcomes included proportions of patients achieving Severity of Alopecia Tool (SALT) scores ≤20 and ≤10, Patient Global Impression of Change (PGI-C) score of 'moderately improved' or 'greatly improved' and eyebrow assessment (EBA) and eyelash assessment (ELA) response (≥2-grade improvement from baseline or normal score in patients with abnormal baseline EBA/ELA).
Results: Mean (SD) ritlecitinib exposure among the 449 de novo patients enrolled was 728.7 (273.81) days. At Month 24 (as observed), 73.5% and 66.4% of patients achieved SALT score ≤20 and ≤10; 82.4% had PGI-C response; 60.8% and 65.7% had EBA and ELA response. 86.1% of patients reported treatment-emergent adverse events (AEs); most were mild or moderate in severity, with the most frequent being positive SARS-CoV-2 test (24.2%), headache (20.8%) and pyrexia (13.0%). Rates of serious AEs, severe AEs and treatment discontinuations were 4.9%, 6.0% and 6.5%, respectively. Herpes zoster infection occurred in six patients, serious infections in four, malignancies (excluding nonmelanoma skin cancer) in three and major adverse cardiovascular events in three.
Conclusions: In patients with AA and ≥25% scalp hair loss, ritlecitinib demonstrated clinical efficacy and had an acceptable safety profile with long-term treatment.
Clinical trial registration: ClinicalTrials.gov NCT04006457.
© 2025 Pfizer Inc and The Author(s). Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Conflict of interest statement
C Tziotzios: speaker for LEO Pharma; principal and chief investigator for Pfizer Inc; consultant for Pfizer Inc. R Sinclair: professional services from AbbVie, Aerotech, Amgen, Arena, Arcutis, AkseBio, AstraZeneca, Ascend, Bayer, Boehringer Ingelheim, BMS, Celgene, Coherus Biosciences, Cutanea, Connect, Dermira, Eli Lilly, Galderma, GSK, Janssen, LEO Pharma, MedImmune, Merck, MSD, Novartis, Oncobiologics, Pfizer Inc, Regeneron, Reistone, Roche, Sanofi, Samson Clinical, Sun Pharma and UCB. A Lesiak: speaker and advisory board member for Novartis, AbbVie, Sanofi, LEO Pharma, Eli Lilly, UCB and Pfizer Inc. S Mehlis: received clinical trial support from AbbVie, Akari, Amgen, AstraZeneca, BMS, ChemoCentryx, Dong‐A ST, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, Menlo, Mayne Pharma, NFlection, Nimbus, Novartis, Pfizer Inc, Regeneron, Sanofi, UCB, Vanda and VYNE; speaker, advisor or consultant for AbbVie, Janssen, Novartis, Dermavant, LEO Pharma and UCB. M Kinoshita‐Ise: clinical trial investigator and speaker for Eli Lilly and Pfizer Inc; clinical trial investigator for BMS and AbbVie. A Tsianakas: clinical trial investigator and speaker for Pfizer Inc. X Luo, EH Law, R Ishowo‐Adejumo, R Wolk, M Sadrarhami and A Lejeune: employees of and hold stock or stock options in Pfizer Inc.
Figures

References
-
- Lauron S, Plasse C, Vaysset M, Pereira B, D'Incan M, Rondepierre F, et al. Prevalence and odds of depressive and anxiety disorders and symptoms in children and adults with alopecia areata: a systematic review and meta‐analysis. JAMA Dermatol. 2023;159:281–288. 10.1001/jamadermatol.2022.6085 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

