Impact of Varying Sleep Pressure on Daytime Sleep Propensity in Healthy Young and Older Adults
- PMID: 39846530
- PMCID: PMC11755553
- DOI: 10.3390/clockssleep7010002
Impact of Varying Sleep Pressure on Daytime Sleep Propensity in Healthy Young and Older Adults
Abstract
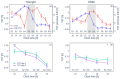
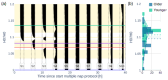
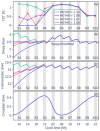
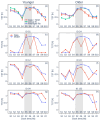
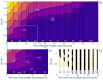
Fixed sleep schedules with an 8 h time in bed (TIB) are used to ensure participants are well-rested before laboratory studies. However, such schedules may lead to cumulative excess wakefulness in young individuals. Effects on older individuals are unknown. We combine modelling and experimental data to quantify the effects of sleep debt on sleep propensity in healthy younger and older participants. A model of arousal dynamics was fitted to sleep data from 22 young (20-31 y.o.) and 26 older (61-82 y.o.) individuals (25 male) undertaking 10 short sleep-wake cycles during a 40 h napping protocol, following >1 week of fixed 8 h TIB schedules. Homeostatic sleep drive at the study start was varied systematically to identify best fits between observed and predicted sleep profiles for individuals and group averages. Daytime sleep duration was the same on the two days of the protocol within the groups but different between the groups (young: 3.14 ± 0.98 h vs. 3.06 ± 0.75 h, older: 2.60 ± 0.98 h vs. 2.37 ± 0.64 h). The model predicted an initial homeostatic drive of 11.2 ± 3.5% (young) and 10.1 ± 3.5% (older) above well-rested. Individual variability in first-day, but not second-day, sleep patterns was explained by the differences in the initial homeostatic drive for both age groups. Our study suggests that both younger and older participants arrive at the laboratory with cumulative sleep debt, despite 8 h TiB schedules, which dissipates after the first four sleep opportunities on the protocol. This has implications for protocol design and the interpretation of laboratory studies.
Keywords: ageing; homeostatic pressure; modelling; napping; sleep; sleep debt; sleep propensity.
Conflict of interest statement
This was not an industry-supported study. The authors of this work declare that there are no conflicts of interest related to this study. While CB is the founder and CEO of PHYSIP SA, no compensation was given to Physip for this research, and Physip did not provide any funding for this research.
Figures
References
-
- Borbely A.A. A Two Process Model of Sleep Regulation. Hum. Neurobiol. 1982;1:195–204. - PubMed
-
- Borbély A.A., Achermann P. Chapter 33—Sleep Homeostasis and Models of Sleep Regulation. In: Kryger M.H., Roth T., Dement W.C., editors. Principles and Practice of Sleep Medicine (Fourth Edition) W.B. Saunders; Philadelphia, PA, USA: 2005. pp. 405–417.
Grants and funding
LinkOut - more resources
Full Text Sources

