Epigenetic Mechanisms of Endocrine-Disrupting Chemicals in Breast Cancer and Their Impact on Dietary Intake
- PMID: 39846533
- PMCID: PMC11755457
- DOI: 10.3390/jox15010001
Epigenetic Mechanisms of Endocrine-Disrupting Chemicals in Breast Cancer and Their Impact on Dietary Intake
Abstract
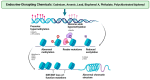
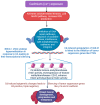
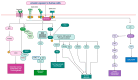
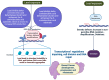
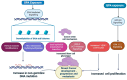
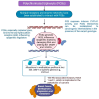
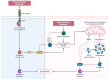
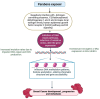
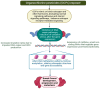
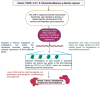
Addressing the consequences of exposure to endocrine-disrupting chemicals (EDCs) demands thorough research and elucidation of the mechanism by which EDCs negatively impact women and lead to breast cancer (BC). Endocrine disruptors can affect major pathways through various means, including histone modifications, the erroneous expression of microRNA (miRNA), DNA methylation, and epigenetic modifications. However, it is still uncertain if the epigenetic modifications triggered by EDCs can help predict negative outcomes. Consequently, it is important to understand how different endocrine disrupters or signals interact with epigenetic modifications and regulate signalling mechanisms. This study proposes that the epigenome may be negatively impacted by several EDCs, such as cadmium, arsenic, lead, bisphenol A, phthalates, polychlorinated biphenyls and parabens, organochlorine, and dioxins. Further, this study also examines the impact of EDCs on lifestyle variables. In breast cancer research, it is essential to consider the potential impacts of EDC exposure and comprehend how EDCs function in tissues.
Keywords: breast cancer; dietary exposure; endocrine disruptors; epigenetic.
Conflict of interest statement
The authors declare that no conflicts of interest.
Figures

References
-
- [(accessed on 23 November 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

