Chlorpyrifos Acts as a Positive Modulator and an Agonist of N-Methyl-d-Aspartate (NMDA) Receptors: A Novel Mechanism of Chlorpyrifos-Induced Neurotoxicity
- PMID: 39846544
- PMCID: PMC11755529
- DOI: 10.3390/jox15010012
Chlorpyrifos Acts as a Positive Modulator and an Agonist of N-Methyl-d-Aspartate (NMDA) Receptors: A Novel Mechanism of Chlorpyrifos-Induced Neurotoxicity
Abstract
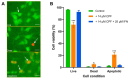
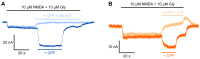
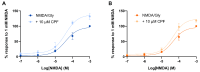
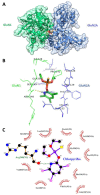
Chlorpyrifos (CPF) is a broad-spectrum organophosphate insecticide. Long-term exposure to low levels of CPF is associated with neurodevelopmental and neurodegenerative disorders. The mechanisms leading to these effects are still not fully understood. Normal NMDA receptor (NMDAR) function is essential for neuronal development and higher brain functionality, while its inappropriate stimulation results in neurological deficits. Thus, the current study aimed to investigate the role of NMDARs in CPF-induced neurotoxicity. We show that NMDARs mediate CPF-induced excitotoxicity in differentiated human fetal cortical neuronal ReNcell CX stem cells. In addition, by using two-electrode voltage clamp electrophysiology of Xenopus oocytes expressing NMDARs, we show CPF potentiation of both GluN1-1a/GluN2A (EC50 ≈ 40 nM) and GluN1-1a/GluN2B (EC50 ≈ 55 nM) receptors, as well as reductions (approximately halved) in the NMDA EC50s and direct activation by 10 μM CPF of both receptor types. In silico molecular docking validated CPF's association with NMDARs through relatively high affinity binding (-8.82 kcal/mol) to a modulator site at the GluN1-GluN2A interface of the ligand-binding domains.
Keywords: NMDA receptors; Xenopus oocytes; chlorpyrifos; neurotoxicity; organophosphate; stem cells; two-electrode voltage clamp.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Saunders M., Magnanti B.L., Carreira S.C., Yang A.L., Alamo-Hernández U., Riojas-Rodriguez H., Calamandrei G., Koppe J.G., von Krauss M.K., Keune H., et al. Chlorpyrifos and neurodevelopmental effects: A literature review and expert elicitation on research and policy. Environ. Health-Glob. 2012;11:S5. doi: 10.1186/1476-069X-11-S1-S5. - DOI - PMC - PubMed
-
- CRD Chemical Regulation Directorate: Plant Protection Products Regulation (EC) No 1107/2009 of March 2016 Concerning Withdrawal of Plant Protection Products in the United Kingdom. [(accessed on 14 June 2024)];2016 Available online: https://secure.pesticides.gov.uk/pestreg/getfile.asp?documentid=29755&ap....
LinkOut - more resources
Full Text Sources

