Advances in magnetic nanoparticles for molecular medicine
- PMID: 39846549
- PMCID: PMC11756346
- DOI: 10.1039/d4cc05167j
Advances in magnetic nanoparticles for molecular medicine
Abstract
Magnetic nanoparticles (MNPs) are highly versatile nanomaterials in nanomedicine, owing to their diverse magnetic properties, which can be tailored through variations in size, shape, composition, and exposure to inductive magnetic fields. Over four decades of research have led to the clinical approval or ongoing trials of several MNP formulations, fueling continued innovation. Beyond traditional applications in drug delivery, imaging, and cancer hyperthermia, MNPs have increasingly advanced into molecular medicine. Under external magnetic fields, MNPs can generate mechano- or thermal stimuli to modulate individual molecules or cells deep within tissue, offering precise, remote control of biological processes at cellular and molecular levels. These unique capabilities have opened new avenues in emerging fields such as genome editing, cell therapies, and neuroscience, underpinned by a growing understanding of nanomagnetism and the molecular mechanisms responding to mechanical and thermal cues. Research on MNPs as a versatile synthetic material capable of engineering control at the cellular and molecular levels holds great promise for advancing the frontiers of molecular medicine, including areas such as genome editing and synthetic biology. This review summarizes recent clinical studies showcasing the classical applications of MNPs and explores their integration into molecular medicine, with the goal of inspiring the development of next-generation MNP-based platforms for disease treatment.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


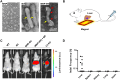
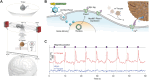








References
-
- Meyers P. H. Nice Jr. C. M. Meckstroth G. R. Becker H. C. Moser P. J. Goldstein M. Pathologic studies following magnetic control of metallic iron particles in the lymphatic and vascular system of dogs as a contrast and isotopic agent. Am. J. Roentgenol., Radium Ther. Nucl. Med. 1966;96:913–921. doi: 10.2214/ajr.96.4.913. - DOI - PubMed
-
- Jaetao J. E. Butler K. S. Adolphi N. L. Lovato D. M. Bryant H. C. Rabinowitz I. Winter S. S. Tessier T. E. Hathaway H. J. Bergemann C. et al., Enhanced leukemia cell detection using a novel magnetic needle and nanoparticles. Cancer Res. 2009;69:8310–8316. doi: 10.1158/0008-5472.CAN-09-1083. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

