Genetic Predisposition to Hippocampal Atrophy and Risk of Amnestic Mild Cognitive Impairment and Alzheimer's Dementia
- PMID: 39846584
- PMCID: PMC11755629
- DOI: 10.3390/geriatrics10010014
Genetic Predisposition to Hippocampal Atrophy and Risk of Amnestic Mild Cognitive Impairment and Alzheimer's Dementia
Abstract
Background: There is a paucity of evidence on the association between genetic propensity for hippocampal atrophy with cognitive outcomes. Therefore, we examined the relationship of the polygenic risk score for hippocampal atrophy (PRShp) with the incidence of amnestic mild cognitive impairment (aMCI) and Alzheimer's disease (AD) as well as the rates of cognitive decline.
Methods: Participants were drawn from the population-based HELIAD cohort. Comprehensive neuropsychological assessments were performed at baseline and at follow-up. PRShp was derived from the summary statistics of a large genome-wide association study for hippocampal volume. Cox proportional hazards models as well as generalized estimating equations (GEEs) were used to evaluate the association of PRShp with the combined incidence of aMCI/AD and cognitive changes over time, respectively. All models were adjusted for age, sex, education, and apolipoprotein E (APOE) genotype.
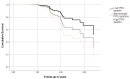
Results: Our analysis included 618 older adults, among whom 73 developed aMCI/AD after an average follow-up of 2.96 ± 0.8 years. Each additional SD of PRShp elevated the relative hazard for incident aMCI/AD by 46%. Participants at the top quartile of PRShp had an almost three times higher risk of converting to aMCI/AD compared to the lowest quartile group. Higher PRShp scores were also linked to steeper global cognitive and memory decline. The impact of PRShp was greater among women and younger adults.
Conclusions: Our findings support the association of PRShp with aMCI/AD incidence and with global cognitive and memory decline over time. The PRS association was sex- and age-dependent, suggesting that these factors should be considered in genetic modelling for AD.
Keywords: Alzheimer’s disease; cognitive decline; hippocampal atrophy; polygenic risk score.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kim H.-R., Jung S.-H., Kim J., Jang H., Kang S.H., Hwangbo S., Kim J.P., Kim S.Y., Kim B., Kim S., et al. Identifying novel genetic variants for brain amyloid deposition: A genome-wide association study in the Korean population. Alzheimers Res. Ther. 2021;13:117. doi: 10.1186/s13195-021-00854-z. - DOI - PMC - PubMed
-
- Armstrong N.M., Dumitrescu L., Huang C.-W., An Y., Tanaka T., Hernandez D., Doshi J., Erus G., Davatzikos C., Ferrucci L., et al. Association of Hippocampal Volume Polygenic Predictor Score with Baseline and Change in Brain Volumes and Cognition Among Cognitively Healthy Older Adults. Neurobiol. Aging. 2020;94:81–88. doi: 10.1016/j.neurobiolaging.2020.05.007. - DOI - PMC - PubMed
-
- Mourtzi N., Charisis S., Tsapanou A., Ntanasi E., Hatzimanolis A., Ramirez A., Heilmann-Heimbach S., Grenier-Boley B., Lambert J.-C., Yannakoulia M., et al. Genetic propensity for cerebral amyloidosis and risk of mild cognitive impairment and Alzheimer’s disease within a cognitive reserve framework. Alzheimers Dement. J. Alzheimers Assoc. 2023;19:3794–3805. doi: 10.1002/alz.12980. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous