Rat as a Predictive Model for Human Clearance and Bioavailability of Monoclonal Antibodies
- PMID: 39846610
- PMCID: PMC11755617
- DOI: 10.3390/antib14010002
Rat as a Predictive Model for Human Clearance and Bioavailability of Monoclonal Antibodies
Abstract
Background: The prediction of human clearance (CL) and subcutaneous (SC) bioavailability is a critical aspect of monoclonal antibody (mAb) selection for clinical development. While monkeys are a well-accepted model for predicting human CL, other preclinical species have been less-thoroughly explored. Unlike CL, predicting the bioavailability of SC administered mAbs in humans remains challenging as contributing factors are not well understood, and preclinical models have not been systematically evaluated.
Methods: Non-clinical and clinical pharmacokinetic (PK) parameters were mined from public and internal sources for rats, cynomolgus monkeys, and humans. Intravenous (IV) and SC PK was determined in Sprague Dawley rats for fourteen mAbs without existing PK data. Together, we obtained cross-species data for 25 mAbs to evaluate CL and SC bioavailability relationships among rats, monkeys, and humans.
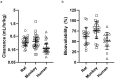
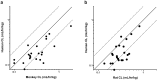
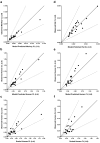
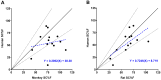
Results: Rat and monkey CL significantly correlated with human CL and supported the use of species-specific exponents for body-weight-based allometric scaling. Notably, rat SC bioavailability significantly correlated with human SC bioavailability, while monkey SC bioavailability did not. Bioavailability also correlated with clearance.
Conclusions: The rat model enables an early assessment of mAb PK properties, allowing discrimination among molecules in the discovery pipeline and prediction of human PK. Importantly, rat SC bioavailability significantly correlated with human SC bioavailability, which has not been observed with other species. Rats are cost-effective and efficient relative to monkeys and provide a valuable tool for pharmacokinetic predictions in therapeutic antibody discovery.
Keywords: allometric scaling; bioavailability; clearance; monoclonal antibody; pharmacokinetics; rat.
Conflict of interest statement
J.D.R., K.M.B., A.M.P., L.H., and P.B.-A. are employees of Eli Lilly and Company and have stock and/or stock interests in Eli Lilly and Company.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

