Phenomenological Modeling of Antibody Response from Vaccine Strain Composition
- PMID: 39846614
- PMCID: PMC11755667
- DOI: 10.3390/antib14010006
Phenomenological Modeling of Antibody Response from Vaccine Strain Composition
Abstract
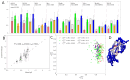
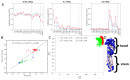
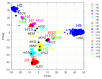
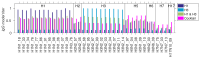
The elicitation of broadly neutralizing antibodies (bnAbs) is a major goal of vaccine design for highly mutable pathogens, such as influenza, HIV, and coronavirus. Although many rational vaccine design strategies for eliciting bnAbs have been devised, their efficacies need to be evaluated in preclinical animal models and in clinical trials. To improve outcomes for such vaccines, it would be useful to develop methods that can predict vaccine efficacies against arbitrary pathogen variants. As a step in this direction, here, we describe a simple biologically motivated model of antibody reactivity elicited by nanoparticle-based vaccines using only antigen amino acid sequences, parametrized with a small sample of experimental antibody binding data from influenza or SARS-CoV-2 nanoparticle vaccinations. Results: The model is able to recapitulate the experimental data to within experimental uncertainty, is relatively insensitive to the choice of the parametrization/training set, and provides qualitative predictions about the antigenic epitopes exploited by the vaccine, which are testable by experiment. For the mosaic nanoparticle vaccines considered here, model results suggest indirectly that the sera obtained from vaccinated mice contain bnAbs, rather than simply different strain-specific Abs. Although the present model was motivated by nanoparticle vaccines, we also apply it to a mutlivalent mRNA flu vaccination study, and demonstrate good recapitulation of experimental results. This suggests that the model formalism is, in principle, sufficiently flexible to accommodate different vaccination strategies. Finally, we show how the model could be used to rank the efficacies of vaccines with different antigen compositions. Conclusions: Overall, this study suggests that simple models of vaccine efficacy parametrized with modest amounts of experimental data could be used to compare the effectiveness of designed vaccines.
Keywords: IgG; coronavirus; hemagglutinin; influenza; simulation; vaccination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Elicitation of Protective Antibodies against 20 Years of Future H3N2 Cocirculating Influenza Virus Variants in Ferrets Preimmune to Historical H3N2 Influenza Viruses.J Virol. 2019 Jan 17;93(3):e00946-18. doi: 10.1128/JVI.00946-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429350 Free PMC article.
-
Comprehensive characterization of the antibody responses to SARS-CoV-2 Spike protein finds additional vaccine-induced epitopes beyond those for mild infection.Elife. 2022 Jan 24;11:e73490. doi: 10.7554/eLife.73490. Elife. 2022. PMID: 35072628 Free PMC article.
-
Mapping of a Novel H3-Specific Broadly Neutralizing Monoclonal Antibody Targeting the Hemagglutinin Globular Head Isolated from an Elite Influenza Virus-Immunized Donor Exhibiting Serological Breadth.J Virol. 2020 Feb 28;94(6):e01035-19. doi: 10.1128/JVI.01035-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31826999 Free PMC article.
-
Strategies for induction of HIV-1 envelope-reactive broadly neutralizing antibodies.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25831. doi: 10.1002/jia2.25831. J Int AIDS Soc. 2021. PMID: 34806332 Free PMC article. Review.
-
Prospects of HA-based universal influenza vaccine.Biomed Res Int. 2015;2015:414637. doi: 10.1155/2015/414637. Epub 2015 Feb 15. Biomed Res Int. 2015. PMID: 25785268 Free PMC article. Review.
References
-
- Chung J.R., Kim S.S., Kondor R.J., Smith C., Budd A.P., Tartof S.Y., Florea A., Talbot H.K., Grijalva C.G., Wernli K.J. Interim Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness–United States, February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:365–370. doi: 10.15585/mmwr.mm7110a1. - DOI - PMC - PubMed
-
- Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T., et al. SARS-CoV-2 MRNa Vaccine Design Enabled by Prototype Pathogen Preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

