Cause of Death Analysis in a 9½-Year-Old with COVID-19 and Dravet Syndrome
- PMID: 39846640
- PMCID: PMC11755659
- DOI: 10.3390/pathophysiology32010003
Cause of Death Analysis in a 9½-Year-Old with COVID-19 and Dravet Syndrome
Abstract
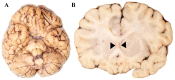
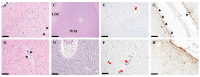
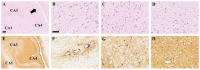
Background: Cause of death analysis is fundamental to forensic pathology. We present the case of a 9½-year-old girl with a genetically confirmed diagnosis of Dravet syndrome who died in her sleep with no evidence of motor seizure. She also had a lifelong history of recurrent pneumonias and, along with her family, had tested positive for COVID-19 10 days before death. Methods: Long-term clinical history of Dravet Syndrome and respiratory infections were obtained from patient's medical charts and radiology reports. A Rapid-Antigen Test was used to confirm SARS-CoV2 infection days prior to death. At autopsy, brain, heart and lung tissues were obtained. Paraffin-embedded tissues were double-stained with H&E, and immunohistochemically stained using various antibodies. Results: Autopsy revealed evidence of previous seizure activity in the brain and cellular interstitial thickening in the lung. The brain showed edema and fibrillary gliosis without neuronal loss in neocortex and hippocampus. The lung showed inflammatory interstitial thickening with histiocytes, megakaryocytes, B-lymphocytes, and T-lymphocytes, including helper/suppressor cells and cytotoxic T-lymphocytes. Diffuse alveolar damage was observed as alveolar flooding with proteinaceous fluid. Conclusions: The cause of death may be attributed to Sudden Unexpected Death in Epilepsy (SUDEP) in Dravet syndrome, sudden death in viral pneumonia, or some combination of the two. When two independent risk factors for sudden unexpected death are identified due to co-pathology, it may not be possible to determine a single cause of death beyond a reasonable doubt.
Keywords: COVID-19; Dravet syndrome; SUDEP; cellular interstitial pneumonia; co-pathology; epilepsy; seizure; sudden unexpected death in epileptic patients; virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Forensic Analysis of 9 Cases of Sudden Unexpected Death in Epilepsy.Fa Yi Xue Za Zhi. 2022 Aug 25;38(4):490-494. doi: 10.12116/j.issn.1004-5619.2020.400616. Fa Yi Xue Za Zhi. 2022. PMID: 36426693 Chinese, English.
-
Autopsy-reported cause of death in a population-based cohort of sudden unexpected death in epilepsy.Epilepsia. 2021 Feb;62(2):472-480. doi: 10.1111/epi.16793. Epub 2021 Jan 5. Epilepsia. 2021. PMID: 33400291
-
Hippocampal abnormalities and seizures: a 16-year single center review of sudden unexpected death in childhood, sudden unexpected death in epilepsy and SIDS.Forensic Sci Med Pathol. 2020 Sep;16(3):423-434. doi: 10.1007/s12024-020-00268-7. Epub 2020 Jul 25. Forensic Sci Med Pathol. 2020. PMID: 32712908
-
Treatments for the prevention of Sudden Unexpected Death in Epilepsy (SUDEP).Cochrane Database Syst Rev. 2020 Apr 2;4(4):CD011792. doi: 10.1002/14651858.CD011792.pub3. Cochrane Database Syst Rev. 2020. PMID: 32239759 Free PMC article.
-
Sudden unexpected death in epilepsy in childhood.Forensic Sci Med Pathol. 2011 Dec;7(4):336-40. doi: 10.1007/s12024-011-9245-6. Epub 2011 May 15. Forensic Sci Med Pathol. 2011. PMID: 21573851 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
