Bone Adaptations to a Whole Body Vibration Protocol in Murine Models of Different Ages: A Preliminary Study on Structural Changes and Biomarker Evaluation
- PMID: 39846667
- PMCID: PMC11755639
- DOI: 10.3390/jfmk10010026
Bone Adaptations to a Whole Body Vibration Protocol in Murine Models of Different Ages: A Preliminary Study on Structural Changes and Biomarker Evaluation
Abstract
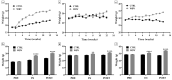
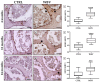
Background/Objectives: Whole body vibration (WBV) is a valuable tool to mitigate physiological adaptations related to age and inactivity. Although significant benefits have been found at the musculoskeletal level, including increased bone mass and reduced muscle atrophy, the underlying biological mechanisms remain largely unknown. Therefore, our study aimed to evaluate the effects of vibratory training on bone tissue in murine models of different age groups by investigating the structural and distribution changes in some crucial biomarkers involved in musculoskeletal homeostasis. Methods: Specifically, 4-, 12-, and 24-month-old mice were trained with a WBV protocol characterized by three series of 2 min and 30 s, interspersed with a recovery period of the same duration, on a 3-weekly frequency for 3 months. At the end of the training, histological and morphometric analyses were conducted, in association with immunohistochemical analysis to investigate changes in the distribution of fibronectin type III domain-containing protein 5 (FNDC5), NADPH oxidase 4 (NOX4), and sirtuin 1 (SIRT1). Results: Our preliminary results showed that WBV improves musculoskeletal health by preserving bone architecture and promoting up-regulation of FNDC5 and SIRT1 and down-regulation of NOX4. Conclusions: Our study confirms vibratory training as a viable alternative to counter musculoskeletal decline in elderly and/or sedentary subjects. Further investigations should be conducted to deepen knowledge in this field and explore the role of other molecular mediators in physiological adaptations to vibration.
Keywords: aging; biomarkers; bone; exercise; musculoskeletal system; physiology; sedentariness; training; vibratory training; whole body vibration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources

