Hypomyelinating Leukodystrophy 14 (HLD14)-Related UFC1 p.Arg23Gln Decreases Cell Morphogenesis: A Phenotype Reversable with Hesperetin
- PMID: 39846712
- PMCID: PMC11755592
- DOI: 10.3390/medicines12010002
Hypomyelinating Leukodystrophy 14 (HLD14)-Related UFC1 p.Arg23Gln Decreases Cell Morphogenesis: A Phenotype Reversable with Hesperetin
Abstract
Introduction: In the central nervous system (CNS), proper interaction between neuronal and glial cells is crucial for the development of mature nervous tissue. Hypomyelinating leukodystrophies (HLDs) are a group of genetic CNS disorders characterized by hypomyelination and/or demyelination. In these conditions, genetic mutations disrupt the biological functions of oligodendroglial cells, which are responsible for wrapping neuronal axons with myelin sheaths. Among these, an amino acid mutation of the ubiquitin-fold modifier conjugating enzyme 1 (UFC1) is associated with HLD14-related disease, characterized by hypomyelination and delayed myelination in the brain. UFC1 is a critical component of the UFMylation system, functioning similarly to E2-conjugating enzymes in the ubiquitin-dependent protein degradation system.
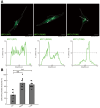
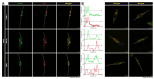
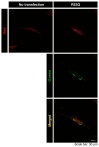
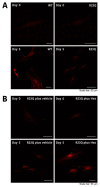
Methodology: We describe how a missense mutation in UFC1 (p.Arg23Gln) leads to the aggregation of UFC1 primarily in lysosomes in FBD-102b cells, which are undergoing oligodendroglial cell differentiation.
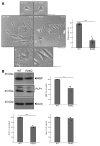
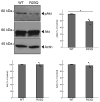
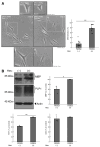
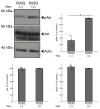
Results: Cells with mutated UFC1 exhibit reduced Akt kinase phosphorylation and reduced expression of differentiation and myelination marker proteins. Consistently, these cells exhibit impaired morphological differentiation with a reduced ability to extend widespread membranes. Interestingly, hesperetin, a citrus flavonoid with known neuroprotective properties, was found to restore differentiation abilities in cells with the UFC1 mutation.
Conclusions: These findings indicate that the HLD14-related mutation in UFC1 causes its lysosomal aggregation, impairing its morphological differentiation. Furthermore, the study highlights potential therapeutic insights into the pathological molecular and cellular mechanisms underlying HLD14 and suggests hesperetin as a promising candidate for treatment.
Keywords: HLD14; UFC1; differentiation; hesperetin; morphogenesis; oligodendrocyte.
Conflict of interest statement
Hiroaki Oizumi, Masahiro Yamamoto, and Katsuya Ohbuchi were employed by Tsumura & Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Hypomyelination Leukodystrophy 16 (HLD16)-Associated Mutation p.Asp252Asn of TMEM106B Blunts Cell Morphological Differentiation.Curr Issues Mol Biol. 2024 Jul 27;46(8):8088-8103. doi: 10.3390/cimb46080478. Curr Issues Mol Biol. 2024. PMID: 39194695 Free PMC article.
-
Hesperetin, a Citrus Flavonoid, Ameliorates Inflammatory Cytokine-Mediated Inhibition of Oligodendroglial Cell Morphological Differentiation.Neurol Int. 2022 May 31;14(2):471-487. doi: 10.3390/neurolint14020039. Neurol Int. 2022. PMID: 35736620 Free PMC article.
-
Claudin-11, a hypomyelinating leukodystrophy 22 (HLD22)-responsible protein, uniquely interacts with shroom-2 to change cell phenotypes.BBA Adv. 2025 Mar 24;7:100159. doi: 10.1016/j.bbadva.2025.100159. eCollection 2025. BBA Adv. 2025. PMID: 40230506 Free PMC article.
-
Molecular Pathogenic Mechanisms of Hypomyelinating Leukodystrophies (HLDs).Neurol Int. 2023 Sep 11;15(3):1155-1173. doi: 10.3390/neurolint15030072. Neurol Int. 2023. PMID: 37755363 Free PMC article. Review.
-
A recurrent de novo HSPD1 variant is associated with hypomyelinating leukodystrophy.Cold Spring Harb Mol Case Stud. 2020 Jun 12;6(3):a004879. doi: 10.1101/mcs.a004879. Print 2020 Jun. Cold Spring Harb Mol Case Stud. 2020. PMID: 32532876 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

