Physico-chemical properties and substrate specificity of α-(1→3)-d-glucan degrading recombinant mutanase from Trichoderma harzianum expressed in Penicillium verruculosum
- PMID: 39846749
- PMCID: PMC11837517
- DOI: 10.1128/aem.00226-24
Physico-chemical properties and substrate specificity of α-(1→3)-d-glucan degrading recombinant mutanase from Trichoderma harzianum expressed in Penicillium verruculosum
Abstract
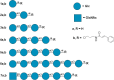
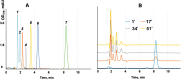
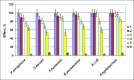
The gene mutAW encoding Trichoderma harzianum fungus mutanase (MutA, GH71 family, α-1,3-glucanase, EC 3.2.1.59) was cloned and heterologously expressed by the highly productive Penicillium verruculosum fungus. P. verruculosum MutA strain secreted crude enzyme preparations with the recombinant MutA content of 40% of the total secreted protein, and the specific activity increased 150 folds compared to that of enzyme preparation obtained by the host strain. Homogeneous MutA had molecular mass of 70 kDa and displayed maximum of the activity on mutan at pH 5.0 and 50°C, with Km and kcat being 1.0 g/L and 30 s-1, respectively. At 40-50°C, the MutA was stable for at least 3 h. Glucose was the main product of long-term mutan hydrolysis. HPLC analysis of hydrolysis product of oligo-α-(1→3)-D-glucosides bearing UV-detectable N-trans-cinnamoyl residue in the aglycon clearly indicated that MutA has an endo-processive hydrolytic mode of action. It was demonstrated that MutA can destroy the polysaccharide matrix of both gram-positive and gram-negative pathogenic bacteria biofilms.
Importance: The manuscript describes the properties of a novel recombinant GH71 mutanase Mut A from Trichoderma harzianum. Gene mutAW encoding mutanase was heterologously expressed in the host strain Penicillium verruculosum B1-537 (ΔniaD). The recipient strain has a high secretory ability and allowed to obtain preparations containing the target recombinant enzyme up to 80% of the total protein pool. MutA exhibited a high activity against mutan and negligible or zero activity toward other types of glucans including α-(1→4)-, β-(1→3)-, β-(1→4)-, and β-(1→6)-glucans. By using a series of synthetic oligo-α-(1→3)-D-glucosides, we demonstrated that MutA is an endo-processive enzyme, which hydrolyzes the internal glucosidic bonds and releases glucose from the reducing end sliding into the non-reducing end. MutA recognizes tetrasaccharide as a minimal substrate and hydrolyzes it to trisaccharide and glucose. The effectiveness of the use of MutA for the destruction of clinical isolates of gram-positive and gram-negative bacteria is also described.
Keywords: Penicillium verruculosum; bacterial biofilms; mutanase; oligo-α-(1→3)-D-glucoside substrates; α-(1→3)-d-glucan; α-1,3-glucanase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Beauvais A, Perlin DS. 2007. Edited by Gadd GM, Watkinson SC, and Dyer P. Role of α(1−3) glucan in Aspergillus fumigatus and other human fungal pathogens in fungi in the environment, p 269–288. Cambridge University Press.
-
- Wiater A, Szczodrak J, Rogalski J. 2004. Hydrolysis of mutan and prevention of its formation in streptococcal films by fungal α-d-glucanases. Process Biochem 39:1481–1489. doi:10.1016/S0032-9592(03)00281-4 - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

