A proteogenomic analysis of the adiposity colorectal cancer relationship identifies GREM1 as a probable mediator
- PMID: 39846783
- PMCID: PMC11754674
- DOI: 10.1093/ije/dyae175
A proteogenomic analysis of the adiposity colorectal cancer relationship identifies GREM1 as a probable mediator
Abstract
Background: Adiposity is an established risk factor for colorectal cancer (CRC). The pathways underlying this relationship, and specifically the role of circulating proteins, are unclear.
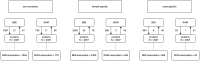
Methods: Utilizing two-sample univariable Mendelian randomization (UVMR), multivariable Mendelian randomization (MVMR), and colocalization, based on summary data from large sex-combined and sex-specific genetic studies, we estimated the univariable associations between: (i) body mass index (BMI) and waist-hip ratio (WHR) and overall and site-specific (colon, proximal colon, distal colon, and rectal) CRC risk, (ii) BMI and WHR and circulating proteins, and (iii) adiposity-associated circulating proteins and CRC risk. We used MVMR to investigate the potential mediating role of adiposity- and CRC-related circulating proteins in the adiposity-CRC association.
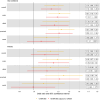
Results: BMI and WHR were positively associated with CRC risk, with similar associations by anatomical tumor site. In total, 6591 adiposity-protein (2628 unique circulating proteins) and 33 protein-CRC (7 unique circulating proteins) associations were identified using UVMR and colocalization. One circulating protein, GREM1, was associated with BMI (only) and CRC outcomes in a manner that was consistent with a potential mediating role in sex-combined and female-specific analyses. In MVMR, adjusting the BMI-CRC association for GREM1, effect estimates were attenuated-suggestive of a potential mediating role-most strongly for the BMI-overall CRC association in women.
Conclusion: Results highlight the impact of adiposity on the plasma proteome and of adiposity-associated circulating proteins on the risk of CRC. Supported by evidence from UVMR and colocalization analyses using cis-single-nucleotide polymorphisms, GREM1 was identified as a potential mediator of the BMI-CRC association, particularly in women.
Keywords: Mendelian randomization; adiposity; colocalization; colorectal cancer; proteome.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Epidemiological Association.
Conflict of interest statement
None declared.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

