Sedation and Ventilator Weaning Bundle and Time to Extubation in Infants With Bronchiolitis: Secondary Analysis of the Sedation AND Weaning in Children (SANDWICH) Trial
- PMID: 39846788
- PMCID: PMC11960679
- DOI: 10.1097/PCC.0000000000003685
Sedation and Ventilator Weaning Bundle and Time to Extubation in Infants With Bronchiolitis: Secondary Analysis of the Sedation AND Weaning in Children (SANDWICH) Trial
Abstract
Objective: The Sedation and Weaning in Children (SANDWICH) trial of a sedation weaning and ventilator liberation bundle had a primary outcome of time to successful extubation, and showed significant but small difference. We explored the impact of the intervention on infants with bronchiolitis.
Design: Post hoc subgroup analysis of a cluster-randomized trial, 2018 to 2019 (ISRCTN16998143).
Patients: Surviving patients with bronchiolitis under 1 year of age in the SANDWICH trial ( n = 784).
Interventions: Nil.
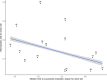
Measurements and main results: Time to successful extubation, and rates of unplanned and failed extubation were compared in patients exposed and not exposed to the intervention. To explore a site-level effect, we tested the correlation between the rate of unplanned and failed extubation at each trial site with the median time to successful extubation at that site. Of 784 patients (48%), 376 were exposed to the intervention. Median (interquartile range [IQR]) time to successful extubation was 69.6 (IQR 50.4-110.4) hours in patients exposed to the intervention and 86.4 (IQR 60-124.8) hours in non-exposed. Exposure to the SANDWICH intervention was associated with a 13% (95% CI, 1%-26%) reduction in time to extubation following adjustment for confounders. Thirty (3.8%) patients experienced unplanned extubation and 112 (14%) failed extubation. Patients who experienced failed extubation had an increased time to successful extubation, which remained significant after adjustment for confounders. At the site level, there was a negative correlation between failed extubation rate and median time to successful extubation (Spearman rho -0.53 [95% CI, -0.8 to -0.08], p = 0.02).
Conclusions: In a secondary analysis of the SANDWICH trial, the subgroup of bronchiolitis patients showed that exposure to the intervention was associated with a clinically significant reduction in time to successful extubation. Although failed extubation was associated with increased duration of ventilation in an individual, sites with higher rates of failed extubation had a lower median duration of ventilation.
Keywords: accidental extubation; bronchiolitis; failed extubation; sedation; ventilation; weaning.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies.
Conflict of interest statement
Dr. Blackwood’s institution received funding from the National Institutes of Health. Dr. Ray’s institution received funding from the National Institute of Health Research (NIHR) Great Ormond Street Hospital Biomedical Research Centre, the U.K. NIHR, the Engineering and Physical Sciences Research Council, and La Roche Ltd. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

References
-
- Miranda M, Ray S, Boot E, et al. : Variation in early pediatric intensive care management strategies and duration of invasive IMV for acute viral bronchiolitis in the United Kingdom: A retrospective multicenter cohort study. Pediatr Crit Care Med. 2023; 24:1010–1021 - PubMed
-
- Bechini A, Salvati C, Bonito B, et al. : Costs and healthcare utilisation due to respiratory syncytial virus disease in paediatric patients in Italy: A systematic review. Public Health. 2024; 227:103–111 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

