First-in-Human Phase I Study of a CD16A Bispecific Innate Cell Engager, AFM24, Targeting EGFR-Expressing Solid Tumors
- PMID: 39846810
- PMCID: PMC11964176
- DOI: 10.1158/1078-0432.CCR-24-1991
First-in-Human Phase I Study of a CD16A Bispecific Innate Cell Engager, AFM24, Targeting EGFR-Expressing Solid Tumors
Abstract
Purpose: Innate immune cell-based therapies have shown promising antitumor activity against solid and hematologic malignancies. AFM24, a bispecific innate cell engager, binds CD16A on NK cells/macrophages and EGFR on tumor cells, redirecting antitumor activity toward tumors. The safety and tolerability of AFM24 were evaluated in this phase I/IIa dose-escalation/dose-expansion study in patients with recurrent or persistent, advanced solid tumors known to express EGFR.
Patients and methods: The main objective in phase I was to determine the MTD and/or recommended phase II dose. The primary endpoint was the incidence of dose-limiting toxicities during the observation period. Secondary endpoints included the incidence of treatment-emergent adverse events and pharmacokinetics.
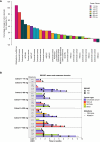
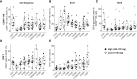
Results: In the dose-escalation phase, 35 patients received AFM24 weekly across seven dose cohorts (14-720 mg). One patient experienced a dose-limiting toxicity of grade 3 infusion-related reaction. Infusion-related reactions were mainly reported after the first infusion; these were manageable with premedication and a gradual increase in infusion rate. Pharmacokinetics was dose-proportional, and CD16A receptor occupancy on NK cells approached saturation between 320 and 480 mg. Paired tumor biopsies demonstrated the activation of innate and adaptive immune responses within the tumor. The best objective response was stable disease in 10/35 patients; four patients had stable disease for 4.3 to 7.1 months.
Conclusions: AFM24 was well tolerated, with 480 mg established as the recommended phase II dose. AFM24 could be a novel therapy for patients with EGFR-expressing solid tumors, with suitable tolerability and appropriate pharmacokinetic properties for further development in combination with other immuno-oncology therapeutics.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A. El-Khoueiry reports grants and nonfinancial support from Affimed during the conduct of the study, as well as personal fees from Roche/Genentech, Eisai, Merck, Bristol Myers Squibb, Exelixis, Qurient, Terumo, Elevar, and Senti Biosciences; grants and personal fees from AstraZeneca; grants from Auransa, Fulgent Genetics, and Astex Pharmaceuticals; and other support from Affimed outside the submitted work. J. Thomas reports personal fees from Coherus BioSciences, Inc. and Kura Oncology, Inc. outside the submitted work. E. Garralda reports grants from Novartis, Roche, Thermo Fisher Scientific, AstraZeneca, Taiho Pharmaceutical, BeiGene, Janssen Pharmaceuticals, and ANAVEON, as well as personal fees from Roche, Ellipses Pharma, Boehringer Ingelheim, Janssen Global Services, Seattle Genetics, Skypta, SOTIO, Sanofi-Aventis, ANAVEON, AbbVie, Astex Therapeutics, Alentis Therapeutics, Marengo Therapeutics, Jiangsu Hengrui, Incyte, Medpace, Medscape, Pfizer, Amgen, Genmab, Greywolf, Gilead Sciences, Daiichi Sankyo, MSD, Roche, Novartis, Seagen, PPD, Aran, TRRF, ESMO, Fundación SEOM, CDDF, Springer Nature, Karger, Doctaforum, Tactics, AEFI, Fundación ECO, MeetingPharma, AstraZeneca, Alcura Health, Horizon CME, and ESO outside the submitted work; in addition, E. Garralda reports employment with NEXT Oncology and stock ownership in 1TRIALSP. J. Koch reports employment with Affimed at the time of the study. E. Rajkovic reports other support from Affimed during the conduct of the study as well as other support from Affimed outside the submitted work. P. Ravenstijn reports employment with Astellas Pharma Europe B.V. P. Nuciforo reports grants from Affimed during the conduct of the study as well as personal fees from Novartis, MSD Oncology, Bayer HealthCare, and Targos Molecular Pathology GmbH outside the submitted work. T.A. Fehniger reports grants and personal fees from Affimed during the conduct of the study as well as grants, personal fees, and other support from Wugen, Orca Bio, and Indapta and grants from HCW Biologics and AI Proteins outside the submitted work; in addition, T.A. Fehniger has a patent for 15/983,275 pending, a patent for 62/963,971 pending, and a patent for PCT/US2019/060005 pending. M.M. Berrien-Elliott reports personal fees from Wugen outside the submitted work. S. Wingert reports personal fees from Affimed outside the submitted work. D. Morales-Espinosa reports other support from Affimed outside the submitted work; in addition, D. Morales-Espinosa is an employee of Affimed GmbH, the sponsor of the study. M. Emig reports personal fees from Affimed GmbH during the conduct of the study as well as personal fees from Affimed GmbH outside the submitted work; in addition, M. Emig reports Affimed stock options. J. Lopez reports grants and personal fees from Roche-Genentech and Basilea Pharmaceutica, grants from MSD and Ex Pharmaceuticals, and personal fees from GSK and Servier outside the submitted work. No disclosures were reported by the other authors.
Figures


References
-
- Wolf NK, Kissiov DU, Raulet DH. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat Rev Immunol 2023;23:90–105. - PubMed
-
- Pinto S, Pahl J, Schottelius A, Carter PJ, Koch J. Reimagining antibody-dependent cellular cytotoxicity in cancer: the potential of natural killer cell engagers. Trends Immunol 2022;43:932–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

