Defining a novel DYRK1A-gp130/IL-6R-pSTAT axis that regulates Th17 differentiation
- PMID: 39846842
- PMCID: PMC11841973
- DOI: 10.1093/immhor/vlae005
Defining a novel DYRK1A-gp130/IL-6R-pSTAT axis that regulates Th17 differentiation
Abstract
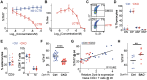
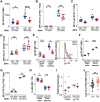
Dysregulated differentiation of naïve CD4+ T cells into T helper 17 (Th17) cells is likely a key factor predisposing to many autoimmune diseases. Therefore, better understanding how Th17 differentiation is regulated is essential to identify novel therapeutic targets and strategies to identify individuals at high risk of developing autoimmunity. Here, we extend our prior work using chemical inhibitors to provide mechanistic insight into a novel regulator of Th17 differentiation, the kinase dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A). We generated a conditional knockout mouse model to validate DYRK1A as a regulator of Th17 differentiation that acts in a dose-dependent fashion at least in part by modulating interleukin (IL)-6 signaling through multiple mechanisms. We identified a new role for DYRK1A in regulating surface expression of IL-6 receptor subunits in naïve CD4+ T cells, consistent with DYRK1A's impact on Th17 differentiation. Physiologic relevance is supported by findings in people with Down syndrome, in which increased expression of DYRK1A, encoded on chromosome 21, is linked to increased IL-6 responsiveness. Our findings highlight DYRK1A as a druggable target of broad therapeutic and prognostic interest in autoimmunity and immune function.
Keywords: DYRK1A; IL-6 signaling; Th17 differentiation; gp130.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
Conflict of interest statement
None declared.
Figures


References
-
- Yasuda K, Takeuchi Y, Hirota K.. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol 2019;41:283–297. - PubMed
-
- Aranda S, Laguna A, de la Luna S.. DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2011;25:449–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

