Prion protein modulation of virus-specific T cell differentiation and function during acute viral infection
- PMID: 39846843
- PMCID: PMC11841969
- DOI: 10.1093/immhor/vlae002
Prion protein modulation of virus-specific T cell differentiation and function during acute viral infection
Abstract
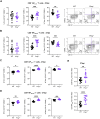
The differentiation and functionality of virus-specific T cells during acute viral infections are crucial for establishing long-term protective immunity. While numerous molecular regulators impacting T cell responses have been uncovered, the role of cellular prion proteins (PrPc) remains underexplored. Here, we investigated the impact of PrPc deficiency on the differentiation and function of virus-specific T cells using the lymphocytic choriomeningitis virus (LCMV) Armstrong acute infection model. Our findings reveal that Prnp-/- mice exhibit a robust expansion of virus-specific CD8+ T cells, with similar activation profiles as wild-type mice during the early stages of infection. However, Prnp-/- mice had higher frequencies and numbers of virus-specific memory CD8+ T cells, along with altered differentiation profiles characterized by increased central and effector memory subsets. Despite similar proliferation rates early during infection, Prnp-/- memory CD8+ T cells had decreased proliferation compared with their wild-type counterparts. Additionally, Prnp-/- mice had higher numbers of cytokine-producing memory CD8+ T cells, indicating a more robust functional response. Furthermore, Prnp-/- mice had increased virus-specific CD4+ T cell responses, suggesting a broader impact of PrPc deficiency on T cell immunity. These results unveil a previously unrecognized role for PrPc in regulating the differentiation, proliferation, and functionality of virus-specific T cells, providing valuable insights into immune system regulation by prion proteins during viral infections.
Keywords: LCMV; T cells; memory T cells; prions; viral infection.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures





References
-
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. - PubMed
-
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. - PubMed
-
- Wherry EJ et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. - PubMed
-
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32AI141346/T32 Microbiology and Infectious Diseases
- R01 AI137239/NH/NIH HHS/United States
- T32 CA009054/CA/NCI NIH HHS/United States
- Musculoskeletal and Skin Diseases
- T32 AI007319/AI/NIAID NIH HHS/United States
- T32 AI141346/AI/NIAID NIH HHS/United States
- National Institute of Arthritis
- NIH
- P30 AR075047/AR/NIAMS NIH HHS/United States
- R01 AI137239/AI/NIAID NIH HHS/United States
- T32AR080622/Interdisciplinary Skin Biology Training
- T32CA009054/CA/NCI NIH HHS/United States
- T32 AR080622/AR/NIAMS NIH HHS/United States
- GM055246/GF/NIH HHS/United States
- R25 GM055246/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

