Synthetic Bilirubin-Based Nanomedicine Protects Against Renal Ischemia/Reperfusion Injury Through Antioxidant and Immune-Modulating Activity
- PMID: 39846887
- PMCID: PMC11912105
- DOI: 10.1002/adhm.202403846
Synthetic Bilirubin-Based Nanomedicine Protects Against Renal Ischemia/Reperfusion Injury Through Antioxidant and Immune-Modulating Activity
Abstract
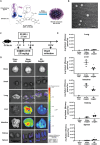
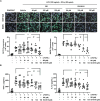
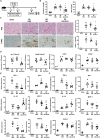
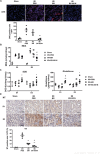
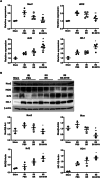
Renal ischemia/reperfusion injury (IRI) is a common form of acute kidney injury. The basic mechanism underlying renal IRI is acute inflammation, where oxidative stress plays an important role. Although bilirubin exhibits potent reactive oxygen species (ROS)-scavenging properties, its clinical application is hindered by problems associated with solubility, stability, and toxicity. In this study, BX-001N, a synthetic polyethylene glycol-conjugated bilirubin 3α nanoparticle is developed and assessed its renoprotective effects in renal IRI. Intravenous administration of BX-001N led to increase uptake in the kidneys with minimal migration to the brain after IRI. Peri-IRI BX-001N administration improves renal function and attenuates renal tissue injury and tubular apoptosis to a greater extent than free bilirubin on day 1 after IRI. BX-001N suppressed renal infiltration of inflammatory cells and reduced expression of TNF-α and MCP-1. Furthermore, BX-001N increases renal tubular regeneration on day 3 and suppresses renal fibrosis on day 28. BX-001N decreases the renal expressions of dihydroethidium, malondialdehyde, and nitrotyrosine after IRI. In conclusion, BX-001N, the first Good Manufacturing Practice-grade synthetic bilirubin-based nanomedicine attenuates acute renal injury and chronic fibrosis by suppressing ROS generation and inflammation after IRI. It shows adequate safety profiles and holds promise as a new therapy for renal IRI.
Keywords: bilirubin nanoparticle; immune modulation; ischemia/reperfusion injury; kidney; reactive oxygen species.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
H.K., S. Jo, D.S., S.M.A., and M.L.K. are employees of Bilix Co., Ltd., which provided BX‐001N for this research. S. Jon is a cofounder and scientific advisory board member of Bilix Co., Ltd. J.Y. received a grant from Bilix Co., Ltd.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

