Surfactin facilitates establishment of Bacillus subtilis in synthetic communities
- PMID: 39846898
- PMCID: PMC11833321
- DOI: 10.1093/ismejo/wraf013
Surfactin facilitates establishment of Bacillus subtilis in synthetic communities
Abstract
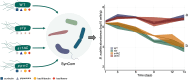
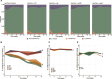
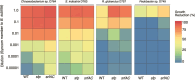
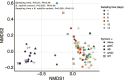
Soil bacteria are prolific producers of a myriad of biologically active secondary metabolites. These natural products play key roles in modern society, finding use as anti-cancer agents, as food additives, and as alternatives to chemical pesticides. As for their original role in interbacterial communication, secondary metabolites have been extensively studied under in vitro conditions, revealing many roles including antagonism, effects on motility, niche colonization, signaling, and cellular differentiation. Despite the growing body of knowledge on their mode of action, biosynthesis, and regulation, we still do not fully understand the role of secondary metabolites on the ecology of the producers and resident communities in situ. Here, we specifically examine the influence of Bacillus subtilis-produced cyclic lipopeptides during the assembly of a bacterial synthetic community, and simultaneously, explore the impact of cyclic lipopeptides on B. subtilis establishment success in a synthetic community propagated in an artificial soil microcosm. We found that surfactin production facilitates B. subtilis establishment success within multiple synthetic communities. Although neither a wild type nor a cyclic lipopeptide non-producer mutant had a major impact on the synthetic community composition over time, both the B. subtilis and the synthetic community metabolomes were altered during co-cultivation. Overall, our work demonstrates the importance of surfactin production in microbial communities, suggesting a broad spectrum of action of this natural product.
Keywords: Bacillus subtilissurfactin; chemical ecology; establishment; secondary metabolites; synthetic community.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Genetic variants of the oppA gene are involved in metabolic regulation of surfactin in Bacillus subtilis.Microb Cell Fact. 2019 Aug 19;18(1):141. doi: 10.1186/s12934-019-1176-z. Microb Cell Fact. 2019. PMID: 31426791 Free PMC article.
-
Production of biosurfactant lipopeptides Iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides.J Microbiol Biotechnol. 2010 Jan;20(1):138-45. J Microbiol Biotechnol. 2010. PMID: 20134245
-
Improvement of surfactin production in Bacillus subtilis using synthetic wastewater by overexpression of specific extracellular signaling peptides, comX and phrC.Biotechnol Bioeng. 2012 Sep;109(9):2349-56. doi: 10.1002/bit.24524. Epub 2012 Apr 24. Biotechnol Bioeng. 2012. PMID: 22511326
-
Biological activity of lipopeptides from Bacillus.Appl Microbiol Biotechnol. 2017 Aug;101(15):5951-5960. doi: 10.1007/s00253-017-8396-0. Epub 2017 Jul 6. Appl Microbiol Biotechnol. 2017. PMID: 28685194 Review.
-
Research advances in the identification of regulatory mechanisms of surfactin production by Bacillus: a review.Microb Cell Fact. 2024 Apr 2;23(1):100. doi: 10.1186/s12934-024-02372-7. Microb Cell Fact. 2024. PMID: 38566071 Free PMC article. Review.
Cited by
-
Characterization of the NRPS operon homolog for surfactin A and surfactin C synthesis in Bacillus spp.Arch Microbiol. 2025 May 29;207(7):161. doi: 10.1007/s00203-025-04341-z. Arch Microbiol. 2025. PMID: 40439731 Free PMC article.
-
Diversification of Lipopeptide Analogues Drives Versatility in Biological Activities.J Agric Food Chem. 2025 Jan 15;73(2):1403-1416. doi: 10.1021/acs.jafc.4c11372. Epub 2025 Jan 6. J Agric Food Chem. 2025. PMID: 39760433 Free PMC article.
-
Plipastatin is a shared good by Bacillus subtilis during combating Fusarium spp.FEMS Microbiol Ecol. 2025 Mar 18;101(4):fiaf020. doi: 10.1093/femsec/fiaf020. FEMS Microbiol Ecol. 2025. PMID: 39999857 Free PMC article.
-
Harnessing the Rhizosphere Microbiome for Selenium Biofortification in Plants: Mechanisms, Applications and Future Perspectives.Microorganisms. 2025 May 28;13(6):1234. doi: 10.3390/microorganisms13061234. Microorganisms. 2025. PMID: 40572122 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

