Early and multiple doses of zoledronate mitigates rebound bone loss following withdrawal of receptor activator of nuclear factor kappa-B ligand inhibition
- PMID: 39846954
- PMCID: PMC11909728
- DOI: 10.1093/jbmr/zjaf008
Early and multiple doses of zoledronate mitigates rebound bone loss following withdrawal of receptor activator of nuclear factor kappa-B ligand inhibition
Abstract
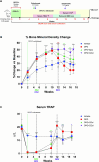
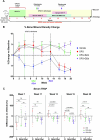
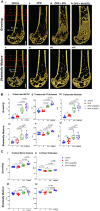
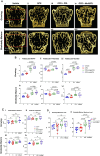
Rebound bone loss following denosumab discontinuation is an important barrier in the effective long-term treatment of skeletal disorders. This is driven by increased osteoclastic bone resorption following the offset of RANKL inhibition, and sequential osteoclast-directed therapy has been utilized to mitigate this. However, current sequential treatment strategies intervene following the offset of RANKL inhibition and this approach fails to consistently prevent bone loss. Our previous work, using a mouse model of denosumab discontinuation, has shown that the processes that drive the rebound phenomenon occur earlier than when bone loss is detected, namely a rise and overshoot in serum tartrate-resistant acid phosphatase (TRAP). We identified that these changes in serum TRAP may provide an earlier window of opportunity to intervene with sequential therapy following RANKL inhibition withdrawal. Here, we show that early treatment with zoledronate (10 mg/kg, 3 wk following the last dose of OPG:Fc), preceding the rise and overshoot in serum TRAP, effectively mitigates rebound bone density loss through preventing the overshoot in serum TRAP. Further, we show that multiple doses of zoledronate (early treatment and during anticipated BMD loss) is superior in consolidating bone density gains made with RANKL inhibition and preventing rebound BMD loss as measured by DXA. Importantly, we demonstrate the efficacy of early and multi-dose zoledronate strategy in preventing bone loss in both growing and skeletally mature mice. MicroCT analysis showed improved trabecular bone structure in both the femur and lumbar vertebrae with zoledronate treatment compared with control. These increases in bone mass translated to increased fracture resistance in skeletally mature mice. This work provides a novel approach of early and multi-dose sequential treatment strategy following withdrawal of RANKL inhibition, contributing valuable insight into the clinical management of patients who discontinue denosumab therapy.
Keywords: denosumab; denosumab discontinuation; osteoporosis; sequential therapy; zoledronate.
Plain language summary
Stopping denosumab leads to loss of bone gained during treatment, due to increased bone resorption when denosumab wears off. Current strategies often fail to prevent this as they cannot stop osteoclasts resorbing bone. Our work using a mouse model has shown that processes that lead to bone loss start earlier than we can detect in the clinic. We show that early and multi-dose zoledronate treatment, another medication used to block osteoclasts, to target these earlier processes can prevent bone loss after stopping denosumab. This approach offers a new strategy for managing bone health in patients stopping denosumab.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
M.M.M. has received honoraria for speaking from Amgen Inc.
Figures



References
MeSH terms
Substances
Grants and funding
- UNSW Sydney University Postgraduate Award
- Australian Government Research Training Program Scholarship
- Australia and New Zealand Bone and Mineral Society and the Endocrine Society of Australia
- R20-05/Ernest Heine Family Foundation and Cancer Council
- American Society of Bone and Mineral Research Rising Star
LinkOut - more resources
Full Text Sources

