A Canadian real-world, multi-center, prospective, observational study assessing the treatment duration, the treatment sequence, and the overall survival for patients treated with endocrine therapy ± targeted therapy in HR + HER2-negative advanced breast cancer
- PMID: 39847203
- PMCID: PMC11930880
- DOI: 10.1007/s10549-024-07580-8
A Canadian real-world, multi-center, prospective, observational study assessing the treatment duration, the treatment sequence, and the overall survival for patients treated with endocrine therapy ± targeted therapy in HR + HER2-negative advanced breast cancer
Abstract
Purpose: Understanding real-world treatment patterns and their effectiveness in HR + HER2- advanced breast cancer (aBC) in Canadian patients.
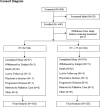
Patient and methods: This was a multi-center, observational, prospective cohort study including men and pre-/peri-/postmenopausal women with HR + HER2- aBC receiving endocrine therapy (ET) or ET + targeted therapy (ET + TT). The primary objective was duration of treatment (DOT) with ET and ET + TT. Sequence of therapies, treatment patterns, and Overall Survival (OS) were also evaluated.
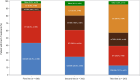
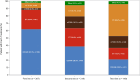
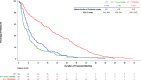
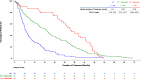
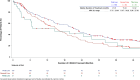
Results: DOT was prolonged in patients receiving ET + TT compared to ET (median DOT: ET + TT 397 days vs ET 192 days; Log-Rank test p value < .0001; HR = 0.66; 95% CI; 0.52, 0.85). An extended DOT was observed in ET + CDK4/6i subgroup when compared to ET (median DOT: ET + CDK4/6i 601 days vs ET 192 days; Log-Rank test p value < .0001). This increase was statistically significant irrespective of line of therapy at baseline (1L: median DOT: ET + CDK4/6i: 649 days vs ET: 217 days, p value = < .0001; 2L: median DOT: ET + CDK4/6i: 487 days vs ET: 203 days, p value = 0.0013; 3L: median DOT: ET + CDK4/6i: 597 days vs ET: 143 days therapy: p value = 0.0006). ET alone and ET + CDK4/6i were the most frequently administered therapies in both 1st (ET alone: 43.5% and ET + CDK4/6i: 43.3%) and 2nd lines (ET alone: 36.3% and ET + CDK4/6i: 24.6%). Among patients who received at least one CDK4/6i in 1st, 2nd, or 3rd line, CDK4/6i were mostly administered in 1st line (61.9%) and 2nd line (38.5%).
Clinicaltrials: gov ID: NCT02753686; Registration Date:20-04-2016.
Conclusion: Results support current treatment recommendations of early introduction of CDK4/6i in HR + /HER2- aBC.
Keywords: Advanced breast cancer; CDK4/6i; Palbociclib; Real-world evidence; Ribociclib; Treatment sequencing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Ana Elisa Lohmann has received honoraria from La Roche-Posay, Novartis, Pfizer, AstraZeneca, Gilead Sciences. Support in kind from Epic Science and Merck. Support for clinical trial from Roche, Novartis, and AstraZeneca. Fellowship support from Eli Lilly and Knight Therapeutics. Jan-Willem Henning has received research grants from AstraZeneca, Pfizer, and Novartis and honoraria from AstraZeneca, Novartis, Pfizer, Gilead, Knight Therapeutics, and University of Toronto. Support as consulting fees and for participation on the advisory board from AstraZeneca, Novartis, Pfizer, Gilead, and Knight Therapeutics. Support for role as chair for Real Canadian Breast Cancer Alliance (Breast CA guideline and advocacy committee). Swati Kulkarni has received honoraria from Novartis. Nadia Califaretti has received honoraria for role as advisory board chair and co-chair as well as in the form of payments made to her medical corporation (no name provided). John Hilton has previously participated in advisory boards/consulted with Novartis on ribociclib and alpelisib. Cristiano Ferrario has received honoraria from AstraZeneca, Bayer, Janssen, Knights Therapeutics, Novartis, Merck, Roche, and Seattle Genetics and support for study conduct from Novartis, AstraZeneca, Bayer, Janssen, Merck, Pfizer, Roche, and Seattle Genetics. Nathaniel Bouganim has received honorarium from AstraZeneca, Novartis, Gilead, and Merck for breast cancer educational activities. Stephen Chia has received support from Novartis during the conduct of the Treat ER + ight trial as well as support for role in the advisory board from Eli Lilly, AstraZeneca, Pfizer, and Merck. Stephanie Guillemette, Ricardo Leite, and Marc-Andre Caron are employees of Novartis Pharmaceuticals Canada Inc. Francois Thireau and Andres Machado are employees of Translational Research in Oncology (TRIO). TRIO was contracted for the conduct of Treat ER + ight trial. Catherine Doyle, Nayyer Iqbal, and Mihaela Mates have no relevant financial or non-financial interests to disclose.
Figures

References
-
- Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. 10.3322/caac.21660 - PubMed
-
- Cancer CCS/ S canadienne du. Breast cancer statistics. Canadian Cancer Society. November 2023. Accessed March 8, 2024. https://cancer.ca/en/cancer-information/cancer-types/breast/statistics
-
- Cancer Tomorrow. Accessed March 8, 2024. https://gco.iarc.who.int/today/
-
- Ruddy KJ, Winer EP (2013) Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann Oncol Off J Eur Soc Med Oncol 24(6):1434–1443. 10.1093/annonc/mdt025 - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer Version 1.2024. Published online January 25, 2024. Accessed March 8, 2024. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

