Antibacterials with Novel Chemical Scaffolds in Clinical Development
- PMID: 39847315
- PMCID: PMC11891108
- DOI: 10.1007/s40265-024-02137-x
Antibacterials with Novel Chemical Scaffolds in Clinical Development
Abstract
The rise of antimicrobial resistance represents a significant global health threat, driven by the diminishing efficacy of existing antibiotics, a lack of novel antibacterials entering the market, and an over- or misuse of existing antibiotics, which accelerates the evolution of resistant bacterial strains. This review focuses on innovative therapies by highlighting 19 novel antibacterials in clinical development as of June 2024. These selected compounds are characterized by new chemical scaffolds, novel molecular targets, and/or unique mechanisms of action, which render their potential to break antimicrobial resistance particularly high. A detailed analysis of the scientific foundations behind each of these compounds is provided, including their pharmacodynamic profiles, current development state, and potential for overcoming existing limitations in antibiotic therapy. By presenting this subset of chemically novel antibacterials, the review highlights the ability to innovate in antibiotic drug development to counteract bacterial resistance and improve treatment outcomes.
© 2025. The Author(s).
Conflict of interest statement
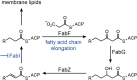
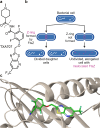
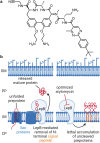
Declarations. Conflict of interest: Alexandra Jana Kohnhäuser is employee of IPG DXTRA (Germany) GmbH, working for dna communications, but the work on this review was neither work-related, nor did she write about content connected to her work-related clients. Dominik Heimann, Daniel Kohnhäuser, Alexandra Jana Kohnhäuser and Mark Brönstrup have declared no conflicts of interest that may be relevant to the contents of this review. Ethics approval: Not applicable. Consent for publication: Not applicable. Consent to participate: Not applicable. Availability of data and materials: Not applicable. Code availability: Not applicable. Funding: Open Access funding enabled and organized by Projekt DEAL. Deutsches Zentrum für Infektionsforschung (TTU09.712). Authors’ contribution: All authors: Design and concept of the entire manuscript. DH: Draft of the introduction, conclusion and chapters about DSTA4637S, zosurabalpin, murepavadin, voxvoganan, PL-18, PLG0206, OMN6 and anti-virulence therapeutics; creation of all figures and the global pipeline table. DK: Draft of chapters about DNA gyrase and topoisomerase IV inhibitors as well as β-lactamase inhibitors; creation of the global pipeline table. AJK: Draft of chapters about afabicin, TXA709, RG6436 and brilacidin. MB: Writing—review and editing, supervision.
Figures










Similar articles
-
Reviewing on AI-Designed Antibiotic Targeting Drug-Resistant Superbugs by Emphasizing Mechanisms of Action.Drug Dev Res. 2025 Feb;86(1):e70066. doi: 10.1002/ddr.70066. Drug Dev Res. 2025. PMID: 39932058 Review.
-
Transition Towards Antibiotic Hybrid Vehicles: The Next Generation Antibacterials.Curr Med Chem. 2022;30(1):104-125. doi: 10.2174/0929867329666220613105424. Curr Med Chem. 2022. PMID: 35702780 Review.
-
Exploring the intricacies of antimicrobial resistance: Understanding mechanisms, overcoming challenges, and pioneering innovative solutions.Eur J Pharmacol. 2025 Jul 5;998:177511. doi: 10.1016/j.ejphar.2025.177511. Epub 2025 Mar 14. Eur J Pharmacol. 2025. PMID: 40090539 Review.
-
A Review of Antibacterial Candidates with New Modes of Action.ACS Infect Dis. 2024 Oct 11;10(10):3440-3474. doi: 10.1021/acsinfecdis.4c00218. Epub 2024 Jul 17. ACS Infect Dis. 2024. PMID: 39018341 Free PMC article. Review.
-
Multi-targeting by monotherapeutic antibacterials.Nat Rev Drug Discov. 2007 Jan;6(1):41-55. doi: 10.1038/nrd2202. Nat Rev Drug Discov. 2007. PMID: 17159922 Review.
Cited by
-
The Changing Landscape of Antibiotic Treatment: Reevaluating Treatment Length in the Age of New Agents.Antibiotics (Basel). 2025 Jul 20;14(7):727. doi: 10.3390/antibiotics14070727. Antibiotics (Basel). 2025. PMID: 40724028 Free PMC article. Review.
-
Zosurabalpin: an antibiotic with a new mechanism of action against carbapenem-resistant Acinetobacter baumannii.Infez Med. 2025 Jun 1;33(2):175-181. doi: 10.53854/liim-3302-3. eCollection 2025. Infez Med. 2025. PMID: 40519345 Free PMC article. Review.
References
-
- Dickter J, Logan C, Taplitz R. Neutropenia and antibiotics: when, what, how and why? Curr Opin Infect Dis. 2023;36:218–27. 10.1097/QCO.0000000000000932. - PubMed
-
- Cossey J, Cote MCB. Evaluation and management of febrile neutropenia in patients with cancer. JAAPA. 2024;37:16–20. 10.1097/01.jaa.0000000000000054. - PubMed
-
- Subramanian AK. Antimicrobial prophylaxis regimens following transplantation. Curr Opin Infect Dis. 2011;24:344–9. 10.1097/qco.0b013e328348b379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

