Aging and inflammation limit the induction of SARS-CoV-2-specific CD8+ T cell responses in severe COVID-19
- PMID: 39847442
- PMCID: PMC11949069
- DOI: 10.1172/jci.insight.180867
Aging and inflammation limit the induction of SARS-CoV-2-specific CD8+ T cell responses in severe COVID-19
Abstract
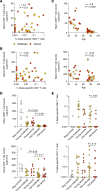
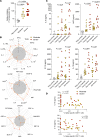
CD8+ T cells are critical for immune protection against severe COVID-19 during acute infection with SARS-CoV-2. However, the induction of antiviral CD8+ T cell responses varies substantially among infected people, and a better understanding of the mechanisms that underlie such immune heterogeneity is required for pandemic preparedness and risk stratification. In this study, we analyzed SARS-CoV-2-specific CD4+ and CD8+ T cell responses in relation to age, clinical status, and inflammation among patients infected primarily during the initial wave of the pandemic in France or Japan. We found that age-related contraction of the naive lymphocyte pool and systemic inflammation were associated with suboptimal SARS-CoV-2-specific CD4+ and, even more evidently, CD8+ T cell immunity in patients with acute COVID-19. No such differences were observed for humoral immune responses targeting the spike protein of SARS-CoV-2. We also found that the proinflammatory cytokine IL-18, concentrations of which were significantly elevated among patients with severe disease, suppressed the de novo induction and memory recall of antigen-specific CD8+ T cells, including those directed against SARS-CoV-2. These results potentially explain the vulnerability of older adults to infections that elicit a profound inflammatory response, exemplified by acute COVID-19.
Keywords: Aging; COVID-19; Cellular immune response; Immunology; T cells.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

