Enhancing the efficacy of near-infrared photoimmunotherapy through intratumoural delivery of CD44-targeting antibody-photoabsorber conjugates
- PMID: 39848206
- PMCID: PMC11795636
- DOI: 10.1016/j.ebiom.2025.105566
Enhancing the efficacy of near-infrared photoimmunotherapy through intratumoural delivery of CD44-targeting antibody-photoabsorber conjugates
Abstract
Background: Photoimmunotherapy (PIT) is a potent modality for cancer treatment. The conventional PIT regimen involves the systemic delivery of an antibody-photoabsorber conjugate, followed by a 24-h waiting period to ensure adequate localisation on the target cells. Subsequent exposure to near-infrared (NIR) light selectively damages the target cells. We aimed to improve the efficacy of PIT in vivo by evaluating the effects of the different routes of conjugate administration on treatment outcomes.
Methods: Subcutaneous Lewis lung carcinoma tumours were established in mice, targeting cluster of differentiation (CD)44 with an anti-CD44 antibody conjugated to IRDye700DX (IR700). The conjugate was administered via the intravenous or intratumoural route followed by the assessment of antibody binding and therapeutic effects of PIT.
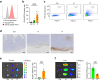
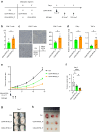
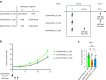
Findings: Compared to intravenous administration, intratumoural delivery of the CD44-IR700 conjugate significantly increased the number of cells binding to the conjugate by >five-fold. This method, combined with NIR light irradiation, halved tumour growth when compared to intravenous delivery. Reducing the interval between intratumoural injection and NIR light exposure to 30 min did not diminish efficacy, thereby demonstrating the feasibility of a 1-h procedure.
Interpretation: Intratumoural administration of the antibody-photoabsorber conjugate enhanced the efficacy of PIT in vivo. A simplified, 1-h procedure involving conjugate tumour injection followed by irradiation emerged as a potent cancer treatment strategy.
Funding: This study was supported by the Japan Society for the Promotion of Science, the Japan Agency for Medical Research and Development, Japan Science and Technology Agency, and the Osaka Medical Research Foundation for Intractable Diseases.
Keywords: Antibody–photoabsorber conjugate; Intratumoural administration; Lung cancer; Photoimmunotherapy.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

