Sprouting sympathetic fibres release CXCL16 and norepinephrine to synergistically mediate sensory neuronal hyperexcitability in a rodent model of neuropathic pain
- PMID: 39848871
- PMCID: PMC11867076
- DOI: 10.1016/j.bja.2024.10.019
Sprouting sympathetic fibres release CXCL16 and norepinephrine to synergistically mediate sensory neuronal hyperexcitability in a rodent model of neuropathic pain
Abstract
Background: Chronic neuropathic pain generally has a poor response to treatment with conventional drugs. Sympathectomy can alleviate neuropathic pain in some patients, suggesting that abnormal sympathetic-somatosensory signaling interactions might underlie some forms of neuropathic pain. The molecular mechanisms underlying sympathetic-somatosensory interactions in neuropathic pain remain obscure.
Methods: Lumbar sympathectomy was performed in spared nerve injury (SNI) mice or rats, and the up-down method was used to measure the mechanical paw withdrawal threshold. Dorsal root ganglia (DRG) injection and perfusion were used to deliver virus or drugs. Methylated RNA immunoprecipitation sequencing, RNA-sequencing, and immunoelectron microscopy were used to identify neurotransmitters.
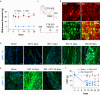
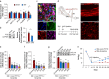
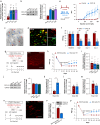
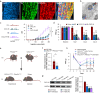
Results: We found that sprouting tyrosine hydroxylase-positive sympathetic fibres in DRG mediated the maintenance of mechanical allodynia after SNI (day 28, P<0.001). We further found that SNI significantly increased the N6-methyladenosine level of CXCL16 messenger RNA (day 28, P<0.001), which was attributable to the reduced N6-methyladenosine demethylase fat mass and obesity-associated protein (P=0.002) and increased interaction with YTHDF1 (P=0.013) in the sympathetic ganglion. Enhanced expression of CXCL16 in the sympathetic ganglia can lead to increases release into the DRG and act synergistically with norepinephrine from sympathetic terminals to enhance DRG neuronal excitability.
Conclusions: Norepinephrine and CXCL16 co-released from sympathetic nerve terminals in the DRG synergistically contribute to maintenance of neuropathic pain in a rodent model.
Keywords: CXC motif chemokine ligand 16; N(6)-methyladenosine; chronic neuropathic pain; norepinephrine; sympathetic ganglion.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interest The authors declare that they have no conflicts of interest.
Figures


References
-
- Attal N., Bouhassira D., Colvin L. Advances and challenges in neuropathic pain: a narrative review and future directions. Br J Anaesth. 2023;131:79–92. - PubMed
-
- Finnerup N.B., Kuner R., Jensen T.S. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101:259–301. - PubMed
-
- Geron M., Tassou A., Scherrer G. Sympathetic yet painful: autonomic innervation drives cluster firing of somatosensory neurons. Neuron. 2022;110:175–177. - PubMed
-
- Borchers A.T., Gershwin M.E. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2014;13:242–265. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

