VCP downstream metabolite glycerol-3-phosphate (G3P) inhibits CD8+T cells function in the HCC microenvironment
- PMID: 39848960
- PMCID: PMC11758394
- DOI: 10.1038/s41392-024-02120-8
VCP downstream metabolite glycerol-3-phosphate (G3P) inhibits CD8+T cells function in the HCC microenvironment
Abstract
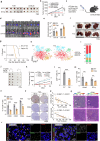
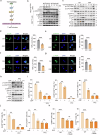
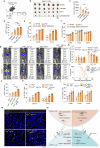
CD8+T cells within the tumor microenvironment (TME) are often functionally impaired, which limits their ability to mount effective anti-tumor responses. However, the molecular mechanisms behind this dysfunction remain incompletely understood. Here, we identified valosin-containing protein (VCP) as a key regulator of CD8+T cells suppression in hepatocellular carcinoma (HCC). Our findings reveal that VCP suppresses the activation, expansion, and cytotoxic capacity of CD8+T cells both in vitro and in vivo, significantly contributing to the immunosuppressive nature of the TME. Mechanistically, VCP stabilizes the expression of glycerol-3-phosphate dehydrogenase 1-like protein (GPD1L), leading to the accumulation of glycerol-3-phosphate (G3P), a downstream metabolite of GPD1L. The accumulated G3P diffuses into the TME and directly interacts with SRC-family tyrosine kinase LCK, a critical component of the T-cell receptor (TCR) signaling pathway in CD8+T cells. This interaction heightens the phosphorylation of Tyr505, a key inhibitory residue, ultimately reducing LCK activity and impairing downstream TCR signaling. Consequently, CD8+T cells lose their functional capacity, diminishing their ability to fight against HCC. Importantly, we demonstrated that targeting VCP in combination with anti-PD1 therapy significantly suppresses HCC tumor growth and restores the anti-tumor function of CD8+T cells, suggesting synergistic therapeutic potential. These findings highlight a previously unrecognized mechanism involving VCP and G3P in suppressing T-cell-mediated immunity in the TME, positioning VCP as a promising upstream target for enhancing immunotherapy in HCC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Anlotinib potentiates anti-PD1 immunotherapy via transferrin receptor-dependent CD8+ T-cell infiltration in hepatocellular carcinoma.Clin Transl Med. 2024 Aug;14(8):e1738. doi: 10.1002/ctm2.1738. Clin Transl Med. 2024. PMID: 39095323 Free PMC article.
-
Targeting MMP9 in CTNNB1 mutant hepatocellular carcinoma restores CD8+ T cell-mediated antitumour immunity and improves anti-PD-1 efficacy.Gut. 2024 May 10;73(6):985-999. doi: 10.1136/gutjnl-2023-331342. Gut. 2024. PMID: 38123979 Free PMC article.
-
CXCL12+ tumor-associated endothelial cells promote immune resistance in hepatocellular carcinoma.J Hepatol. 2025 Apr;82(4):634-648. doi: 10.1016/j.jhep.2024.09.044. Epub 2024 Oct 9. J Hepatol. 2025. PMID: 39393439
-
In Situ Vaccination as a Strategy to Modulate the Immune Microenvironment of Hepatocellular Carcinoma.Front Immunol. 2021 May 7;12:650486. doi: 10.3389/fimmu.2021.650486. eCollection 2021. Front Immunol. 2021. PMID: 34025657 Free PMC article. Review.
-
Tumor-associated macrophages and CD8+ T cells: dual players in the pathogenesis of HBV-related HCC.Front Immunol. 2024 Oct 10;15:1472430. doi: 10.3389/fimmu.2024.1472430. eCollection 2024. Front Immunol. 2024. PMID: 39450177 Free PMC article. Review.
Cited by
-
Research progress of CD73-adenosine signaling regulating hepatocellular carcinoma through tumor microenvironment.J Exp Clin Cancer Res. 2025 May 26;44(1):161. doi: 10.1186/s13046-025-03416-5. J Exp Clin Cancer Res. 2025. PMID: 40420185 Free PMC article. Review.
References
-
- Vogel, A., Meyer, T., Sapisochin, G., Salem, R. & Saborowski, A. Hepatocellular carcinoma. Lancet400, 1345–1362 (2022). - PubMed
-
- Sangro, B. et al. A randomized, multicenter, phase 3 study of nivolumab vs sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J. Clin. Oncol. 34, TPS4147-TPS4147 (2016).
-
- Yang, C. et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol.20, 203–222 (2023). - PubMed
-
- Zhu, A. X. et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol.19, 940–952 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

