KorB switching from DNA-sliding clamp to repressor mediates long-range gene silencing in a multi-drug resistance plasmid
- PMID: 39849085
- PMCID: PMC11790492
- DOI: 10.1038/s41564-024-01915-3
KorB switching from DNA-sliding clamp to repressor mediates long-range gene silencing in a multi-drug resistance plasmid
Abstract
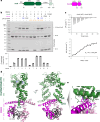
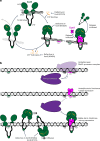
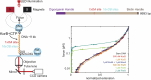
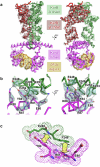
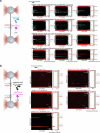
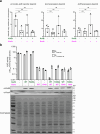
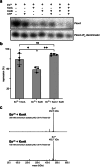
Examples of long-range gene regulation in bacteria are rare and generally thought to involve DNA looping. Here, using a combination of biophysical approaches including X-ray crystallography and single-molecule analysis for the KorB-KorA system in Escherichia coli, we show that long-range gene silencing on the plasmid RK2, a source of multi-drug resistance across diverse Gram-negative bacteria, is achieved cooperatively by a DNA-sliding clamp, KorB, and a clamp-locking protein, KorA. We show that KorB is a CTPase clamp that can entrap and slide along DNA to reach distal target promoters up to 1.5 kb away. We resolved the tripartite crystal structure of a KorB-KorA-DNA co-complex, revealing that KorA latches KorB into a closed clamp state. DNA-bound KorA thus stimulates repression by stalling KorB sliding at target promoters to occlude RNA polymerase holoenzymes. Together, our findings explain the mechanistic basis for KorB role switching from a DNA-sliding clamp to a co-repressor and provide an alternative mechanism for long-range regulation of gene expression in bacteria.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








Similar articles
-
Flexibility in repression and cooperativity by KorB of broad host range IncP-1 plasmid RK2.J Mol Biol. 2005 Jun 3;349(2):302-16. doi: 10.1016/j.jmb.2005.03.062. Epub 2005 Apr 8. J Mol Biol. 2005. PMID: 15890197
-
Conserved C-terminal region of global repressor KorA of broad-host-range plasmid RK2 is required for co-operativity between KorA and a second RK2 global regulator, KorB.J Mol Biol. 1999 Jun 4;289(2):211-21. doi: 10.1006/jmbi.1999.2761. J Mol Biol. 1999. PMID: 10366500
-
Dissection of the switch between genes for replication and transfer of promiscuous plasmid RK2: basis of the dominance of trfAp over trbAp and specificity for KorA in controlling the switch.J Mol Biol. 1997 Feb 7;265(5):507-18. doi: 10.1006/jmbi.1996.0747. J Mol Biol. 1997. PMID: 9048945
-
Genes encoding the pKM101 conjugal mating pore are negatively regulated by the plasmid-encoded KorA and KorB proteins.J Bacteriol. 1996 Aug;178(15):4392-9. doi: 10.1128/jb.178.15.4392-4399.1996. J Bacteriol. 1996. PMID: 8755865 Free PMC article.
-
Molecular Mechanisms of Transcription Initiation at gal Promoters and their Multi-Level Regulation by GalR, CRP and DNA Loop.Biomolecules. 2015 Oct 16;5(4):2782-807. doi: 10.3390/biom5042782. Biomolecules. 2015. PMID: 26501343 Free PMC article. Review.
Cited by
-
Expanding the diversity of bacterial DNA partitioning: A CTP-independent ParABS system for plasmid partitioning in Streptomyces.Proc Natl Acad Sci U S A. 2025 Jul 8;122(27):e2406398122. doi: 10.1073/pnas.2406398122. Epub 2025 Jul 2. Proc Natl Acad Sci U S A. 2025. PMID: 40601632 Free PMC article.
-
Evolution of a Plasmid Regulatory Circuit Ameliorates Plasmid Fitness Cost.bioRxiv [Preprint]. 2024 May 30:2024.02.05.579024. doi: 10.1101/2024.02.05.579024. bioRxiv. 2024. Update in: Mol Biol Evol. 2025 Apr 1;42(4):msaf062. doi: 10.1093/molbev/msaf062. PMID: 38370613 Free PMC article. Updated. Preprint.
-
Evolution of a Plasmid Regulatory Circuit Ameliorates Plasmid Fitness Cost.Mol Biol Evol. 2025 Apr 1;42(4):msaf062. doi: 10.1093/molbev/msaf062. Mol Biol Evol. 2025. PMID: 40138356 Free PMC article.
References
-
- Schoenfelder, S. & Fraser, P. Long-range enhancer–promoter contacts in gene expression control. Nat. Rev. Genet.20, 437–455 (2019). - PubMed
-
- Kim, S., Beltran, B., Irnov, I. & Jacobs-Wagner, C. Long-distance cooperative and antagonistic RNA polymerase dynamics via DNA supercoiling. Cell179, 106–119.e16 (2019). - PubMed
-
- Wang, Z. & Dröge, P. Long-range effects in a supercoiled DNA domain generated by transcription in vitro. J. Mol. Biol.271, 499–510 (1997). - PubMed
-
- Bignell, C. R., Haines, A. S., Khare, D. & Thomas, C. M. Effect of growth rate and incC mutation on symmetric plasmid distribution by the IncP-1 partitioning apparatus. Mol. Microbiol.34, 205–216 (1999). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

