Chronic inflammation in post-acute sequelae of COVID-19 modulates gut microbiome: a review of literature on COVID-19 sequelae and gut dysbiosis
- PMID: 39849406
- PMCID: PMC11756069
- DOI: 10.1186/s10020-024-00986-6
Chronic inflammation in post-acute sequelae of COVID-19 modulates gut microbiome: a review of literature on COVID-19 sequelae and gut dysbiosis
Abstract
Background: Long COVID or Post-acute sequelae of COVID-19 is an emerging syndrome, recognized in COVID-19 patients who suffer from mild to severe illness and do not recover completely. Most studies define Long COVID, through symptoms like fatigue, brain fog, joint pain, and headache prevailing four or more weeks post-initial infection. Global variations in Long COVID presentation and symptoms make it challenging to standardize features of Long COVID. Long COVID appears to be accompanied by an auto-immune multi-faceted syndrome where the virus or viral antigen persistence causes continuous stimulation of the immune response, resulting in multi-organ immune dysregulation.
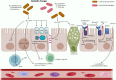
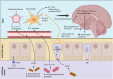
Main text: This review is focused on understanding the risk factors of Long COVID with a special emphasis on the dysregulation of the gut-brain axis. Two proposed mechanisms are discussed here. The first mechanism is related to the dysfunction of angiotensin-converting enzyme 2 receptor due to Severe Acute Respiratory Syndrome Corona Virus 2 infection, leading to impaired mTOR pathway activation, reduced AMP secretion, and causing dysbiotic changes in the gut. Secondly, gut-brain axis dysregulation accompanied by decreased production of short-chain fatty acids, impaired enteroendocrine cell function, and increased leakiness of the gut, which favors translocation of pathogens or lipopolysaccharide in circulation causing the release of pro-inflammatory cytokines. The altered Hypothalamic-Pituitary-Adrenal axis is accompanied by the reduced level of neurotransmitter, and decreased stimulation of the vagus nerve, which may cause neuroinflammation and dysregulation of serum cortisol levels. The dysbiotic microbiome in Long COVID patients is characterized by a decrease in beneficial short chain fatty acid-producing bacteria (Faecalibacterium, Ruminococcus, Dorea, and Bifidobacterium) and an increase in opportunistic bacteria (Corynebacterium, Streptococcus, Enterococcus). This dysbiosis is transient and may be impacted by interventions including probiotics, and dietary supplements.
Conclusions: Further studies are required to understand the geographic variation, racial and ethnic differences in phenotypes of Long COVID, the influence of viral strains on existing and emerging phenotypes, to explore long-term effects of gut dysbiosis, and gut-brain axis dysregulation, as well as the potential role of diet and probiotics in alleviating those symptoms.
Keywords: COVID-19; Gut-brain axis; Inflammation; Long COVID; Microbiota; PASC.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Altmann DM, Whettlock EM, Liu S, Arachchillage DJ, Boyton RJ. The immunology of long COVID. Nat Rev Immunol. 2023;2023:1–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

