Functional heterogeneity of mesenchymal stem cells and their therapeutic potential in the K18-hACE2 mouse model of SARS-CoV-2 infection
- PMID: 39849557
- PMCID: PMC11756204
- DOI: 10.1186/s13287-024-04086-4
Functional heterogeneity of mesenchymal stem cells and their therapeutic potential in the K18-hACE2 mouse model of SARS-CoV-2 infection
Abstract
Background: Despite many years of investigation into mesenchymal stem cells (MSCs) and their potential for treating inflammatory conditions such as COVID-19, clinical outcomes remain variable due to factors like donor variability, different tissue sources, and diversity within MSC populations. Variations in MSCs' secretory and proliferation profiles, and their proteomic and transcriptional characteristics significantly influence their therapeutic potency, highlighting the need for enhanced characterization methods to better predict their efficacy. This study aimed to evaluate the biological characteristics of MSCs from different tissue origins, selecting the most promising line for further validation in a K18-hACE2 mouse model of SARS-CoV-2 infection.
Methods: We studied nine MSC lines sourced from either bone marrow (hBMMSC), dental pulp (hDPMSC), or umbilical cord tissue (hUCMSC). The cells were assessed for their proliferative capacity, immunophenotype, trilineage differentiation, proteomic profile, and in vitro immunomodulatory potential by co-culture with activated lymphocytes. The most promising MSC line was selected for further experimental validation using the K18-hACE2 mouse model of SARS-CoV-2 infection.
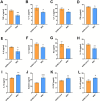
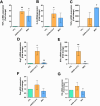
Results: The analyzed cells met the minimum criteria for defining MSCs, including the expression of surface molecules and differentiation capacity, showing genetic stability and proliferative potential. Proteomic analysis revealed distinct protein profiles that correlate with the tissue origin of MSCs. The immunomodulatory response exhibited variability, lacking a discernible pattern associated with their origin. In co-culture assays with lymphocytes activated with anti-CD3/CD28 beads, all MSC lines demonstrated the ability to inhibit TNF-α, to induce TGF-β and Indoleamine 2,3-dioxygenase (IDO), with varying degrees of inhibition observed for IFN-γ and IL-6, or induction of IL-10 expression. A module of proteins was found to statistically correlate with the potency of IL-6 modulation, leading to the selection of one of the hUCMSCs as the most promising line. Administration of hUCMSC to SARS-CoV-2-infected K18 mice expressing hACE2 was effective in improving lung histology and modulating of a panel of cytokines.
Conclusions: Our study assessed MSCs derived from various tissues, uncovering significant variability in their characteristics and immunomodulatory capacities. Particularly, hUCMSCs demonstrated potential in mitigating lung pathology in a SARS-CoV-2 infection model, suggesting their promising therapeutic efficacy.
Keywords: COVID-19; Heterogeneity; Immunomodulation; K18-hACE2; Mesenchymal stem cells.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of Hospital São Rafael (CAAE: 09803819.30000.0048, titled: “Avaliação do potencial terapêutico de celulas-tronco mesenquimais para uso clínico”, date of approval: 04/02/2019). All tissue donors or their legal guardians gave written informed consent for participation and publication of this study. The study involving mice was approved by the Institutional Animal Care and Use Committee (IACUC) of the Federal University of Minas Gerais (Comissão de Ética no Uso de Animais – CEUA). The project, titled ‘Implementação e caracterização de modelo experimental de COVID-19 em camundongos transgênicos K18-hACE2,’ was approved on September 13, 2021, under protocol number 191/2021. Name of the institutional approval committee or unit: Aprovado pela Comissão de Ética no uso de animais (CEUA) da Universidade Federal de Minas Gerais. The approval number: CEUA 191/2021. Date of approval: 13/09/2021. Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
The K18-Human ACE2 Transgenic Mouse Model Recapitulates Non-severe and Severe COVID-19 in Response to an Infectious Dose of the SARS-CoV-2 Virus.J Virol. 2022 Jan 12;96(1):e0096421. doi: 10.1128/JVI.00964-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668775 Free PMC article.
-
A human-ACE2 knock-in mouse model for SARS-CoV-2 infection recapitulates respiratory disorders but avoids neurological disease associated with the transgenic K18-hACE2 model.mBio. 2025 May 14;16(5):e0072025. doi: 10.1128/mbio.00720-25. Epub 2025 Apr 24. mBio. 2025. PMID: 40272151 Free PMC article.
-
SARS-CoV-2 Causes Lung Infection without Severe Disease in Human ACE2 Knock-In Mice.J Virol. 2022 Jan 12;96(1):e0151121. doi: 10.1128/JVI.01511-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668780 Free PMC article.
-
Mesenchymal Stem Cell-Based COVID-19 Therapy: Bioengineering Perspectives.Cells. 2022 Jan 29;11(3):465. doi: 10.3390/cells11030465. Cells. 2022. PMID: 35159275 Free PMC article. Review.
-
Exploring the Immunomodulatory Aspect of Mesenchymal Stem Cells for Treatment of Severe Coronavirus Disease 19.Cells. 2022 Jul 12;11(14):2175. doi: 10.3390/cells11142175. Cells. 2022. PMID: 35883618 Free PMC article. Review.
Cited by
-
Mesenchymal stromal cell secretome reduces lung injury and thrombo-inflammation induced by SARS-CoV-2 spike protein.Stem Cell Res Ther. 2025 Jul 1;16(1):324. doi: 10.1186/s13287-025-04472-6. Stem Cell Res Ther. 2025. PMID: 40597299 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- INOVA Novos talentos 2021/Foundation for Scientific and Technological Development in Health
- IDOR-Serrapilheira-COVI-19/Instituto Serrapilheira
- Laboratórios de Campanha 0494/20 01.20.0026.00 and FINEP UFMG-NB3 nº 1139/20/Financiadora de Estudos e Projetos
- 465656/2014-5, 408124/2021-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 26/010.001488/2019; E-26/210.338/2022/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

