Outcomes in stage IIA versus stage IIB/III in the PALLAS trial [ABCSG-42/AFT-05/PrE0109/BIG-14-13])
- PMID: 39849600
- PMCID: PMC11761723
- DOI: 10.1186/s13058-024-01941-3
Outcomes in stage IIA versus stage IIB/III in the PALLAS trial [ABCSG-42/AFT-05/PrE0109/BIG-14-13])
Abstract
Background: The PALLAS trial investigated the addition of palbociclib to standard adjuvant endocrine therapy to reduce breast cancer recurrence. This pre-specified analysis was conducted to determine whether adjuvant palbociclib benefited patients diagnosed with lower risk stage IIA disease compared to those with higher stage disease.
Methods: PALLAS was an international, multicenter, randomized, open-label, phase III trial, representing a public-private partnership between Pfizer, the Austrian Breast Cancer Study Group, and the U.S. ALLIANCE Foundation. Patients diagnosed with stage II-III, hormone-receptor-positive, HER2/neu negative breast cancer within 12 months of diagnosis had completed all definitive therapy aside from endocrine therapy (started within 6 months prior to study entry) were eligible. All patients were required to submit a formalin-fixed paraffin-embedded (FFPE) tumor block. Patients were randomly assigned 1:1 to receive standard adjuvant endocrine therapy (of physicians' choice) for at least 5 years with or without 2 years of palbociclib, administered orally at a starting dose of 125 mg daily, given for 21 days followed by a 7-day break.
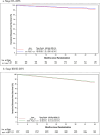
Results: A total of 5,796 patients with HR + /HER2- early breast cancer (including 1,010 with stage IIA) were enrolled. Median follow-up was 50 months for stage IIA patients and 43.1 months overall. In the stage IIA cohort, 4-year iDFS in the palbociclib arm was 92.9% versus 92.1% for ET alone (HR 0.75, 95%CI 0.48-1.19, p = 0.23). There was no differential benefit by histologic grade, chemotherapy receipt, age, or anatomic/clinical risk. Additionally, no benefit to palbociclib was seen in this cohort in invasive breast cancer-free survival (iBCFS), locoregional relapse-free survival (LRFS), distant relapse-free survival (DRFS), or overall survival (OS). For the stage IIB/III patients, 4-year iDFS was 85.3% for palbociclib + ET versus 83.6% for ET alone (HR 0.91, 95% CI 0.77-1.07, p = 0.24).
Conclusions and relevance: While there were substantial differences in outcome for stage IIA versus IIB/III patients at 4 years of follow-up, the addition of 2 years of palbociclib did not improve outcomes for patients, regardless of stage.
Trial registration: ClinicalTrials.gov number NCT02513394 Registered 30 Jul 2015.
Keywords: Adjuvant; CDK4/6 inhibitor; Endocrine; Stage II breast cancer.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: In the U.S., the study protocol was approved by the institutional review board and the Advarra Protocol Approval number is Pro00034499. Outside the U.S., the trial was approved by respectively responsible institutional review boards and ethics committees. All analyzed patients provided written informed consent, and the trial was performed in strict accordance with ICH GCP guidelines (ClinicalTrials.gov identifier: NCT02513394, EudraCT 2014-005181-30). Competing interests: Institutional research grants from Novartis, Pfizer, Genentech, and Neogenomics. The authors declare no competing interests.
Figures

References
-
- Johnston SRD, Toi M, O’Shaughnessy J, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77–90. - DOI - PMC - PubMed
-
- Administration USFaD: FDA approves ribociclib with an aromatase inhibitor and ribociclib and letrozole co-pack for early high-risk breast cancer. www.fda.gov, U.S Food and Drug Administration, 2024.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

