Engineered ipilimumab variants that bind human and mouse CTLA-4
- PMID: 39849917
- PMCID: PMC11776466
- DOI: 10.1080/19420862.2025.2451296
Engineered ipilimumab variants that bind human and mouse CTLA-4
Abstract
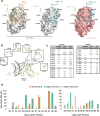
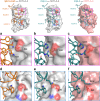
Testing of candidate monoclonal antibody therapeutics in preclinical models is an essential step in drug development. Identification of antibody therapeutic candidates that bind their human targets and cross-react to mouse orthologs is often challenging, especially for targets with low sequence homology. In such cases, surrogate antibodies that bind mouse orthologs must be used. The antibody 9D9, which binds mouse CTLA-4, is a commonly used surrogate for CTLA-4 checkpoint blockade studies in mouse cancer models. In this work, we reveal that 9D9 has significant biophysical dissimilarities to therapeutic CTLA-4 antibodies. The 9D9-mCTLA4 complex crystal structure was determined and shows that the surrogate antibody binds an epitope distinct from ipilimumab and tremelimumab. In addition, while ipilimumab has pH-independent binding to hCTLA-4, 9D9 loses binding to mCTLA-4 at physiologically relevant acidic pH ranges. We used phage and yeast display to engineer ipilimumab to bind mouse CTLA-4 with single-digit nM affinity from an initial state with no apparent binding. The engineered variants showed pH-independent and cross-reactive binding to both mouse and human CTLA-4. Crystal structures of a variant in complex with both mouse and human CTLA-4 confirmed that it targets an equivalent epitope as ipilimumab. These cross-reactive ipilimumab variants may facilitate improved translatability and future mechanism-of-action studies for anti-CTLA-4 targeting in murine models.
Keywords: Antibody engineering; CTLA-4; immune checkpoint; phage display; species cross reactivity; yeast display.
Conflict of interest statement
The sequences of the mipi variants are provided in this work.
Figures





References
-
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi:10.1084/jem.20130579. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
