Exo- and Endo-1,5-α-L-Arabinanases and Prebiotic Arabino-Oligosaccharides Production
- PMID: 39849927
- PMCID: PMC11813348
- DOI: 10.4014/jmb.2412.12052
Exo- and Endo-1,5-α-L-Arabinanases and Prebiotic Arabino-Oligosaccharides Production
Abstract
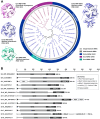
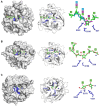
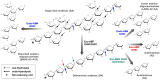
There is growing interest in pentose-based prebiotic oligosaccharides as alternatives to traditional hexose-based prebiotics. Among these, arabino-oligosaccharides (AOS), derived from the enzymatic hydrolysis of arabinan polymers, have gained significant attention. AOS can selectively stimulate the growth of beneficial gut bacteria, including Bifidobacterium and Bacteroides species, and contribute to health-benefit functions such as blood sugar control, positioning AOS as a promising synbiotic candidate. For the industrial production of AOS, the development of efficient enzymatic processes is essential, with exo- and endo-1,5-α-L-arabinanases (exo- and endo-ABNs) playing a crucial catalytic role. Most ABNs belong to the glycoside hydrolase (GH) family 43, characterized by a five-bladed β-propeller fold structure. These enzymes hydrolyze internal α-1,5-L-arabinofuranosidic linkages, producing AOS with varying degrees of polymerization. Some ABNs GH43 were known to exhibit exo-type hydrolytic modes of action, producing specific AOS products such as arabinotriose. Additionally, exo-ABNs from GH93, which feature a six-bladed β-propeller fold, exclusively release arabinobiose through their exo-type catalytic mechanism. This review represents the first comprehensive analysis of exo- and endo-ABNs, offering scientific insights into their biotechnological potential for AOS production. It systematically compares enzyme classification, structural differences, catalytic mechanisms, paving the way for innovative applications in health, food, and pharmaceutical industries.
Keywords: 5-α-L-arabinanase (endo-ABN); Endo-1; arabino-oligosaccharides (AOS); enzymatic production; exo-1,5-α-L-arabinanase (exo-ABN); structure-function relationship.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures
Similar articles
-
Functional Characterization of Endo- and Exo-Hydrolase Genes in Arabinan Degradation Gene Cluster of Bifidobacterium longum subsp. suis.Int J Mol Sci. 2024 Mar 9;25(6):3175. doi: 10.3390/ijms25063175. Int J Mol Sci. 2024. PMID: 38542148 Free PMC article.
-
Mechanistic strategies for catalysis adopted by evolutionary distinct family 43 arabinanases.J Biol Chem. 2014 Mar 14;289(11):7362-73. doi: 10.1074/jbc.M113.537167. Epub 2014 Jan 27. J Biol Chem. 2014. PMID: 24469445 Free PMC article.
-
Structures of endo-1,5-α-L-arabinanase mutants from Bacillus thermodenitrificans TS-3 in complex with arabino-oligosaccharides.Acta Crystallogr F Struct Biol Commun. 2018 Dec 1;74(Pt 12):774-780. doi: 10.1107/S2053230X18015947. Epub 2018 Nov 26. Acta Crystallogr F Struct Biol Commun. 2018. PMID: 30511671 Free PMC article.
-
Oligosaccharides Derived from Red Seaweed: Production, Properties, and Potential Health and Cosmetic Applications.Molecules. 2018 Sep 25;23(10):2451. doi: 10.3390/molecules23102451. Molecules. 2018. PMID: 30257445 Free PMC article. Review.
-
Inulin Potential for Enzymatic Obtaining of Prebiotic Oligosaccharides.Crit Rev Food Sci Nutr. 2016 Aug 17;56(11):1893-902. doi: 10.1080/10408398.2013.807220. Crit Rev Food Sci Nutr. 2016. PMID: 25746219 Review.
References
-
- Rajagopalan G, Krishnan C. In: Next Generation Biomanufacturing Technologies. Rathinam NK, Sani R, editors. ACS Publications, American Chemical Society; Washington D.C: 2019. Functional oligosaccharides: production and action; pp. 155–180.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

