Exploring the Proteomic Landscape and Immunomodulatory Functions of Edwardsiella piscicida Derived Extracellular Vesicles
- PMID: 39849936
- PMCID: PMC11813346
- DOI: 10.4014/jmb.2410.10001
Exploring the Proteomic Landscape and Immunomodulatory Functions of Edwardsiella piscicida Derived Extracellular Vesicles
Abstract
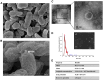
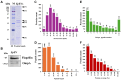
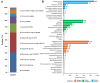
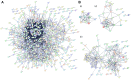
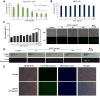
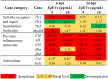
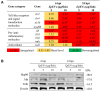
Extracellular vesicles (EVs) have garnered attention in research for their potential as biochemical transporters and immune modulators, crucial for regulating the host immune system. The present study was conducted to isolate and characterize EVs from Gram negative bacteria Edwardsiella piscicida (EpEVs) and investigate their proteomic profile and immune responses. Isolation of EpEVs was carried out using ultracentrifugation method. Transmission electron microscopy results confirmed the spherical shape of EpEVs. The average size and zeta potential were 85.3 ± 1.8 nm and -8.28 ± 0.41 mV, respectively. EpEVs consisted of 1,487 distinct proteins. Subcellular localization analysis revealed that "cell" and "cell part" were the most predominant areas for protein localization. Proteins associated with virulence, along with several chaperones that facilitate protein folding and stability, were also present. No toxicity was detected when EpEVs were treated to fathead minnow (FHM) cells up to 100 μg/ml. Fluorescent-labeled EpEVs showed cellular internalization in FHM cells at 24 h post treatment (hpt). In-vitro gene expression in Raw 264.7 cells showed upregulation of interleukin (Il)6, Il1β, and interferon (Ifn)β with simultaneous upregulation of anti-inflammatory Il10. In vivo, gene expression revealed that except for heat shock protein (hsp)70, all other genes were upregulated suggesting that EpEVs induced the expression of immune-related genes. Western blot analysis showed increased protein levels of tumor necrosis factor (Tnf)α in EpEVs-treated spleen tissue of zebrafish. Our results confirm that EpEVs can be successfully isolated using the ultracentrifugation method. Furthermore, exploring immunomodulatory mechanism of EpEVs is essential for their potential use as novel therapeutics in fish medicine.
Keywords: Edwardsiella piscicida; Raw 264.7 cells; Zebrafish (Danio rerio); extracellular vesicles; immunomodulation; proteomic analysis.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures
References
-
- Wen M, Wang J, Ou Z, Nie G, Chen Y, Li M, et al. Bacterial extracellular vesicles: a position paper by the microbial vesicles task force of the Chinese society for extracellular vesicles. Interdisciplinary Medicine. 2023;1:e20230017. doi: 10.1002/INMD.20230017. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

