bFGF-Chitosan "brain glue" promotes functional recovery after cortical ischemic stroke
- PMID: 39850018
- PMCID: PMC11755050
- DOI: 10.1016/j.bioactmat.2024.12.017
bFGF-Chitosan "brain glue" promotes functional recovery after cortical ischemic stroke
Abstract
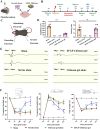
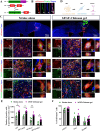
The mammalian brain has an extremely limited ability to regenerate lost neurons and to recover function following ischemic stroke. A biomaterial strategy of slowly-releasing various regeneration-promoting factors to activate endogenous neurogenesis represents a safe and practical neuronal replacement therapy. In this study, basic fibroblast growth factor (bFGF)-Chitosan gel is injected into the stroke cavity. This approach promotes the proliferation of vascular endothelial cell, the formation of functional vascular network, and the final restoration of cerebral blood flow. Additionally, bFGF-Chitosan gel activates neural progenitor cells (NPCs) in the subventricular zone (SVZ), promotes the NPCs' migration toward the stroke cavity and differentiation into mature neurons with diverse cell types (inhibitory gamma-aminobutyric acid neurons and excitatory glutamatergic neuron) and layer architecture (superficial cortex and deep cortex). These new-born neurons form functional synaptic connections with the host brain and reconstruct nascent neural networks. Furthermore, synaptogenesis in the stroke cavity and Nestin lineage cells respectively contribute to the improvement of sensorimotor function induced by bFGF-Chitosan gel after ischemic stroke. Lastly, bFGF-Chitosan gel inhibits microglia activation in the peri-infarct cortex. Our findings indicate that filling the stroke cavity with bFGF-Chitosan "brain glue" promotes angiogenesis, endogenous neurogenesis and synaptogenesis to restore function, offering innovative ideas and methods for the clinical treatment of ischemic stroke.
Keywords: Angiogenesis; Ischemic stroke; Neural circuit reconstruction; New-born neurons; Synaptogenesis; bFGF-Chitosan gel.
© 2024 The Authors.
Conflict of interest statement
The authors affirm that they have no known financial or interpersonal conflicts that could have appeared to have an impact on the research presented in this study.
Figures







References
-
- Campbell B.C.V., De Silva D.A., Macleod M.R., et al. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019;5(1):70. - PubMed
-
- Moretti A., Ferrari F., Villa R.F. Neuroprotection for ischaemic stroke: current status and challenges. Pharmacol. Ther. 2015;146:23–34. - PubMed
-
- Tang L., Yin Y., Liu H., et al. Blood-brain barrier-penetrating and lesion-targeting nanoplatforms inspired by the pathophysiological features for synergistic ischemic stroke therapy. Adv. Mater. 2024;36(21) - PubMed
LinkOut - more resources
Full Text Sources

