Current update on the neurological manifestations of long COVID: more questions than answers
- PMID: 39850323
- PMCID: PMC11755773
- DOI: 10.17179/excli2024-7885
Current update on the neurological manifestations of long COVID: more questions than answers
Abstract
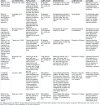
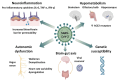
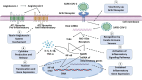
Since the outbreak of the COVID-19 pandemic, there has been a global surge in patients presenting with prolonged or late-onset debilitating sequelae of SARS-CoV-2 infection, colloquially termed long COVID. This narrative review provides an updated synthesis of the latest evidence on the neurological manifestations of long COVID, discussing its clinical phenotypes, underlying pathophysiology, while also presenting the current state of diagnostic and therapeutic approaches. Approximately one-third of COVID-19 survivors experience prolonged neurological sequelae that persist for at least 12-months post-infection, adversely affecting patients' quality of life. Core neurological manifestations comprise fatigue, post-exertional malaise, cognitive impairment, headache, lightheadedness ('brain fog'), sleep disturbances, taste or smell disorders, dysautonomia, anxiety, and depression. Some of these features overlap substantially with those reported in post-intensive-care syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, and postural-orthostatic-tachycardia syndrome. Advances in data-driven research utilizing electronic-health-records combined with machine learning and artificial intelligence have propelled the identification of long COVID sub-phenotypes. Furthermore, the evolving definitions reflect the dynamic conceptualization of long COVID in both research and clinical contexts. Although the underlying pathophysiology remains incompletely elucidated, neuroinflammatory responses, endotheliopathy, and metabolic imbalances, rather than direct viral neuroinvasion, are implicated in neurological sequelae. Genetic susceptibility has also emerged as a potential risk factor. While major limitations remain with existing definitions, collaborative strategies to standardize diagnostic approaches are needed. Current therapeutic paradigms advocate for multimodal approaches, integrating pharmacological and non-pharmacological interventions along with comprehensive rehabilitation programs. Although preliminary evidence of therapeutic efficacy has been provided by a number of clinical trials, methodological constraints limit the generalizability of this evidence. Preventive measures, notably vaccination, have proven integral for reducing the global burden of long COVID. Considering the healthcare and socioeconomic repercussions incurred by long COVID worldwide, international collaborative initiatives are warranted to address the remaining challenges in diagnosing and managing patients presenting with neurological sequelae. See also the graphical abstract(Fig. 1).
Keywords: COVID-19; PACS; SARS-CoV-2; brain fog; long COVID; long-haul.
Copyright © 2024 Stefanou et al.
Figures





References
-
- Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome coronavirus (MERS) outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
