Daily oscillation of the excitation/inhibition ratio is disrupted in two mouse models of autism
- PMID: 39850357
- PMCID: PMC11754079
- DOI: 10.1016/j.isci.2024.111494
Daily oscillation of the excitation/inhibition ratio is disrupted in two mouse models of autism
Erratum in
-
Erratum: Daily oscillation of the excitation/inhibition ratio is disrupted in two mouse models of autism.iScience. 2025 Feb 4;28(2):111917. doi: 10.1016/j.isci.2025.111917. eCollection 2025 Feb 21. iScience. 2025. PMID: 39991545 Free PMC article.
Abstract
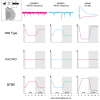
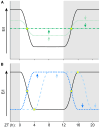
Alterations to the excitation/inhibition (E/I) ratio are postulated to underlie behavioral phenotypes in autism spectrum disorder (ASD) patients and mouse models. However, in wild type mice the E/I ratio is not constant, but instead oscillates across the 24-h day. Therefore, we tested whether E/I regulation, rather than the overall E/I ratio, is disrupted in two ASD-related mouse lines: Fmr1 KO and BTBR, models of syndromic and idiopathic ASD, respectively. The E/I ratio is dysregulated in both models, but in different ways: the oscillation is lost in Fmr1 KO and reversed in BTBR mice. Phenotypes in both models associate with differences the timing of excitatory and inhibitory synaptic transmission and endocannabinoid signaling compared to wild type mice, but not with altered sleep. These findings raise the possibility that ASD-related phenotypes may be produced by a mismatch between E/I and behavioral state, rather than alterations to overall E/I levels per se.
Keywords: Neuroscience; Sensory neuroscience.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Maenner M.J., Warren Z., Williams A.R., Amoakohene E., Bakian A.V., Bilder D.A., Durkin M.S., Fitzgerald R.T., Furnier S.M., Hughes M.M., et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2020. MMWR. Surveill. Summ. 2023;72:1–14. - PMC - PubMed
-
- Goel A., Cantu D.A., Guilfoyle J., Chaudhari G.R., Newadkar A., Todisco B., de Alba D., Kourdougli N., Schmitt L.M., Pedapati E., et al. Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat. Neurosci. 2018;21:1404–1411. doi: 10.1038/s41593-018-0231-0. - DOI - PMC - PubMed
-
- Berto S., Treacher A.H., Caglayan E., Luo D., Haney J.R., Gandal M.J., Geschwind D.H., Montillo A.A., Konopka G. Association between resting-state functional brain connectivity and gene expression is altered in autism spectrum disorder. Nat. Commun. 2022;13:3328. doi: 10.1038/s41467-022-31053-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

