Single-cell RNA sequencing elucidates cellular plasticity in esophageal small cell carcinoma following chemotherapy treatment
- PMID: 39850495
- PMCID: PMC11754407
- DOI: 10.3389/fgene.2024.1477705
Single-cell RNA sequencing elucidates cellular plasticity in esophageal small cell carcinoma following chemotherapy treatment
Abstract
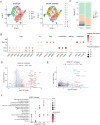
Small cell carcinoma of the esophagus (SCCE) is a rare and aggressively progressing malignancy that presents considerable clinical challenges.Although chemotherapy can effectively manage symptoms during the earlystages of SCCE, its long-term effectiveness is notably limited, with theunderlying mechanisms remaining largely undefined. In this study, weemployed single-cell RNA sequencing (scRNA-seq) to analyze SCCE samplesfrom a single patient both before and after chemotherapy treatment. Our analysisrevealed significant cellular plasticity and alterations in the tumormicroenvironment's cellular composition. Notably, we observed an increase intumor cell diversity coupled with reductions in T cells, B cells, and myeloid-likecells. The pre-treatment samples predominantly featured carcinoma cells in amiddle transitional state, while post-treatment samples exhibited an expandedpresence of cells in terminal, initial-to-terminal (IniTerm), and universally alteredstates. Further analysis highlighted dynamic interactions between tumor cells andimmune cells, with significant changes detected in key signaling pathways, suchas TIGIT-PVR and MDK-SDC4. This study elucidates the complex dynamics of cellplasticity in SCCE following chemotherapy, providing new insights and identifyingpotential therapeutic targets to enhance treatment efficacy.
Keywords: cell plasticity; chemotherapy; single-cell RNA sequencing; small cell carcinoma of the esophagus; tumor microenvironment.
Copyright © 2025 Zhang, Gao, Qiu, Cao, Zhang, Qin, Yang, Wang, Yang, Li and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- GBD 2017 Oesophageal Cancer Collaborators (2020). The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterology. Hepatology 5 (6), 582–597. 10.1016/S2468-1253(20)30007-8 - DOI - PMC - PubMed
-
- Barnestein R., Galland L., Kalfeist L., Ghiringhelli F., Ladoire S., Limagne E. (2022). Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. Oncoimmunology 11 (1), 2120676. 10.1080/2162402X.2022.2120676 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

