MicroRNA-155 as Biomarker and Its Diagnostic Value in Breast Cancer: A Systematic Review
- PMID: 39850528
- PMCID: PMC11750751
- DOI: 10.14740/wjon1955
MicroRNA-155 as Biomarker and Its Diagnostic Value in Breast Cancer: A Systematic Review
Abstract
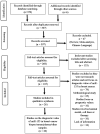
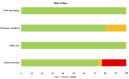
The investigation of microRNAs (miRNAs) for the purpose of identifying biomarkers and new treatments for breast cancer has been gaining traction from scientists in recent years. Of all the miRNAs, miR-155 has been reportedly involved in breast cancer development as it regulates various cellular processes such as glucose uptake, proliferation, metastasis, and migration. Various efforts have been done towards researching miR-155 as a biomarker in breast cancer; however, the results were varied. The objective of the current systematic review is to compile and summarize information regarding miR-155 as a potential diagnostic biomarker for breast cancer. All eligible studies were found from SCOPUS and PubMed databases. Out of the 376 potential eligible records, only 26 original articles were selected for further assessment according to inclusion and exclusion criteria. The expressions of miR-155 in serum, plasma, biopsy, urine, nipple aspirate fluid, serum exosomes, and peripheral blood mononuclear cells were recorded and analyzed. Besides that, the expression of miR-155 was also correlated to clinicopathological features in breast cancer patients. The area under the curve (AUC) values from receiver operating characteristic (ROC) analysis used to evaluate diagnostic sensitivity and specificity of miR-155 as a diagnostic biomarker were also recorded. The limitations such as the small sampling size, the unemployment of internal controls for quantitative real-time polymerase chain reaction (RT-qPCR), and inconsistency of sensitivity as well as specificity values of miR-155 as a biomarker have been discussed. The present study proposed that miR-155 is a good diagnostic biomarker for breast cancer; however, further clinical research is required to assess the validity of miR-155 as a potential biomarker to translate the research outcomes into clinical practice.
Keywords: Biomarker; Breast cancer; Diagnostics; MiR-155; MicroRNA; Systematic review.
Copyright 2025, Yeo et al.
Conflict of interest statement
The authors declare that there is no competing financial interests or personal relationships that can appear to influence the work presented in this article.
Figures

References
-
- World Health Organisation (WHO). Estimated age-standardized incidence rates (world) in 2020, Breast, Female, All Ages, Asia. 2020.
-
- Bernardi D, Macaskill P, Pellegrini M, Valentini M, Fanto C, Ostillio L, Tuttobene P. et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol. 2016;17(8):1105–1113. doi: 10.1016/S1470-2045(16)30101-2. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
