Effects and Mechanisms of Silibinin on Influenza A/H1N1 Pathogenesis in a Mouse Model
- PMID: 39850538
- PMCID: PMC11756948
- DOI: 10.1155/jotm/6618423
Effects and Mechanisms of Silibinin on Influenza A/H1N1 Pathogenesis in a Mouse Model
Abstract
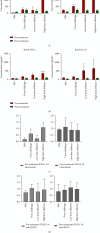
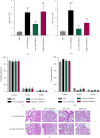
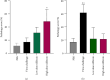
Silymarin is a polyphenolic flavonoid extracted from milk thistle. It has potent immunomodulatory effects and can inhibit the replication of influenza A virus (IAV). The present study aimed to determine the inflammatory and anti-inflammatory cytokine secretion patterns in mice before and after silibinin treatment. For this, bronchoalveolar lavage (BAL) fluids were collected from the thoracic cavity 5 days after the intervention, and viral quantification was performed using TaqMan Real-time PCR. Enzyme-linked immunosorbent assay (ELISA) was used to evaluate IFN-γ and IL-10 levels in serum and BAL samples. Finally, pathological damage to lung tissue was assessed by pathologists. The results reveal that silibinin pretreatment exhibits a dose-dependent immunomodulatory effect on IFN-γ and IL-10 levels. After the virus challenge, silibinin reduced immune cell infiltration in mouse BAL fluid. These data similarly suggest a remarkable immunomodulatory effect of silibinin. Silibinin also decreased lung damage following the virus challenge in the post-treatment group, but its lung protective properties seem to be due to a different mechanism than when it was administered before infection. Finally, high doses of silibinin (post-treatment) significantly reduced viral load in BAL fluid compared to the virus challenge group. These results support the idea that therapies aimed at moderating immune and inflammatory responses are essential to decrease the mortality rate caused by IAV infection. Silibinin has strong immunomodulatory properties, can inhibit IAV infection, and reduces lung tissue damage in a dose-dependent manner.
Keywords: immunomodulatory effect; influenza A virus (IAV); lung damage; silibinin; silymarin.
Copyright © 2025 Mohsen Keshavarz et al. Journal of Tropical Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention.Carcinogenesis. 1999 Nov;20(11):2101-8. doi: 10.1093/carcin/20.11.2101. Carcinogenesis. 1999. PMID: 10545412
-
Mahuang Xixin Fuzi decoction protects the BALB/c-nude mice infected with influenza A virus by reducing inflammatory cytokines storm and weakly regulating SIgA immune response.J Ethnopharmacol. 2023 Mar 25;304:116070. doi: 10.1016/j.jep.2022.116070. Epub 2022 Dec 19. J Ethnopharmacol. 2023. PMID: 36549371
-
Progesterone-Based Contraceptives Reduce Adaptive Immune Responses and Protection against Sequential Influenza A Virus Infections.J Virol. 2017 Mar 29;91(8):e02160-16. doi: 10.1128/JVI.02160-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28179523 Free PMC article.
-
Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells.Prostate. 2002 Nov 1;53(3):211-7. doi: 10.1002/pros.10146. Prostate. 2002. PMID: 12386921
-
Silibinin as a major component of milk thistle seed provides promising influences against diabetes and its complications: a systematic review.Naunyn Schmiedebergs Arch Pharmacol. 2024 Oct;397(10):7531-7549. doi: 10.1007/s00210-024-03172-x. Epub 2024 May 27. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38801454
Cited by
-
Polyphenols as Antiviral Agents: Their Potential Against a Range of Virus Types.Nutrients. 2025 Jul 16;17(14):2325. doi: 10.3390/nu17142325. Nutrients. 2025. PMID: 40732950 Free PMC article. Review.
References
-
- Fraschini F., Demartini G., Esposti D. Pharmacology of Silymarin. Clinical Drug Investigation . 2002;22(1):51–65. doi: 10.2165/00044011-200222010-00007. - DOI
-
- Sabbaghi A., Miri S. M., Keshavarz M., Mahooti M., Zebardast A., Ghaemi A. Role of γδ T Cells in Controlling Viral Infections with a Focus on Influenza Virus: Implications for Designing Novel Therapeutic Approaches. Virology Journal . 2020;17(1):174–218. doi: 10.1186/s12985-020-01449-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

