Adverse drug events (ADEs) risk signal mining related to eculizumab based on the FARES database
- PMID: 39850567
- PMCID: PMC11754194
- DOI: 10.3389/fphar.2024.1440907
Adverse drug events (ADEs) risk signal mining related to eculizumab based on the FARES database
Erratum in
-
Corrigendum: Adverse drug events (ADEs) risk signal mining related to eculizumab based on the FARES database.Front Pharmacol. 2025 Feb 7;16:1558930. doi: 10.3389/fphar.2025.1558930. eCollection 2025. Front Pharmacol. 2025. PMID: 39989898 Free PMC article.
Abstract
Introduction: Eculizumab is a C5 complement inhibitor approved by the FDA for the targeted treatment of four rare diseases, paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), generalized myasthenia gravis (gMG), and aquaporin-4 immunoglobulin G-positive optic neuromyelitis optica spectrum disorders (AQP4-IgG+NMOSD). The current study was conducted to assess real-world adverse events (AEs) associated with eculizumab through data mining of the FDA Adverse Event Reporting System (FAERS).
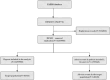
Methods: Disproportionality analyses, including Reporting Ratio Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-Item Gamma Poisson Shrinker (MGPS) algorithms were used to quantify the signals of eculizumab-associated AEs.
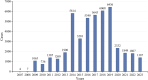
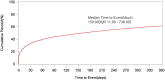
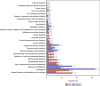
Results: A total of 46,316 eculizumab-related ADEs reports were identified by analyzing 19,418,776 reports in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) database. A total of 461 PTs were identified as satisfying by all four algorithms. These PTs reported adverse reactions consistent with the specifications, such as fatigue, nasopharyngitis, meningococcal infection, fever, and anemia. Some PTs, such as aplastic anemia, gene mutation, mastication disorder, kidney fibrosis, BK virus infection, abnormal neutrophil count, C3 glomerulopathy, neuroblastoma, and glomerulonephritis membranoproliferative, were also detected outside the instructions. The median time to onset of eculizumab adverse events was 159 days (interquartile range [IQR] 11∼738 days). In addition, at the PT level, 51 PTs were determined to have an imbalance in the occurrence of ADEs between the sexes.
Conclusion: These findings provide valuable insights into the occurrence of ADEs following the use of eculizumab and could support clinical monitoring and risk identification efforts.
Keywords: ADRM; FAERS; adverse drug events; adverse drug reaction monitoring; eculizumab.
Copyright © 2025 Wang, Bao, Hu, Xu, Gao, Wang, Zhao, Fu, Meng and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Chen C., Ziyan J. I. N., Xinyi X. U., Zhang C., Bin W. U., Ting X. U. (2021). Safety comparison of axitinib and everolimus in the treatment of renal cancer:analysis of adverse events based on pharmacovigilance database. Anti-tumor Pharm. 11 (04), 385–393. 10.3969/j.issn.2095-1264.2021.04.01 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

