A Novel Homozygous BMP15 Mutation Causes Ovarian Dysgenesis and Primary Amenorrhea
- PMID: 39850788
- PMCID: PMC11756294
- DOI: 10.1210/jendso/bvae221
A Novel Homozygous BMP15 Mutation Causes Ovarian Dysgenesis and Primary Amenorrhea
Abstract
Context: Despite a growing number of studies, the genetic etiology in many cases of ovarian dysgenesis is incompletely understood.
Objectives: This work aimed to study the genetic etiology causing absence of spontaneous pubertal development, hypergonadotropic hypogonadism, and primary amenorrhea in 2 sisters.
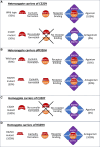
Methods: Whole-exome sequencing was performed on DNA extracted from peripheral lymphocytes of 2 Palestinian sisters born to consanguineous parents. Following a BMP15 variant identification, confirming genetic segregation studies were performed in family members. Three-dimensional (3D) modeling for BMP15 dimer and BMP15-GDF-9 heterodimer were followed by functional studies in human ovarian COV434 granulosa cells cotransfected with plasmid harboring either the variant or a wild-type (WT) control, and a second plasmid harboring a luciferase-reporter-gene with a BMP-responsive element.
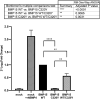
Results: A novel homozygous c.G959A/p.C320Y BMP15 mutation was identified in both sisters, and segregated with the disease in the family. By 3D-structure modeling, the mutations were predicted to damage a cysteine-knot motif, disrupt BMP15 dimerization, and severely impair activation of the BMP pathway. The homologous mutation C53Y occurring and identified spontaneously in sheep results in sterility in homozygotes, mimicking the human phenotype here. A 3.8-fold decrease in BMP15 signaling was observed in vitro in cells expressing the homozygous BMP15 mutant when compared to the WT control.
Conclusion: The novel homozygous missense C320Y mutation is the first homozygous human BMP15 variant causing impaired signaling ability, which correlates with the predicted 3D-structural changes leading to ovarian dysgenesis. The homologous mutation in sheep mimics the human phenotype by infertility. Beyond genetic counseling, and considering ovarian preservation, the ovine model enables further elucidation and interventions in the BMP signaling.
Keywords: BMP15; infertility; ovarian dysgenesis; primary amenorrhea; primary ovarian insufficiency.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019;22(4):403‐411. - PubMed
-
- De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376(9744):911‐921. - PubMed
-
- Vitt UA, Mazerbourg S, Klein C, Hsueh AJ. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod. 2002;67(2):473‐480. - PubMed
-
- Grøndahl ML, Borup R, Vikeså J, Ernst E, Andersen CY, Lykke-Hartmann K. The dormant and the fully competent oocyte: comparing the transcriptome of human oocytes from primordial follicles and in metaphase II. Mol Hum Reprod. 2013;19(9):600‐617. - PubMed
-
- Kristensen SG, Andersen K, Clement CA, Franks S, Hardy K, Andersen CY. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol Hum Reprod. 2014;20(4):293‐308. - PubMed
LinkOut - more resources
Full Text Sources
