Investigating the Effect of Syringe Infiltration on Nicotiana tabacum (Tobacco)
- PMID: 39850806
- PMCID: PMC11752493
- DOI: 10.1021/acsagscitech.4c00170
Investigating the Effect of Syringe Infiltration on Nicotiana tabacum (Tobacco)
Abstract
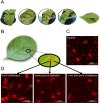
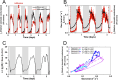
Plant infiltration techniques, particularly agroinfiltration, have transformed plant science and biotechnology by enabling transient gene expression for genetic engineering of plants or genomic studies. Recently, the use of infiltration has expanded to introduce nanomaterials and polymers in plants to enable nonnative functionalities. Despite its wide use, the impact of the infiltration process per se on plant physiology needs to be better understood. This study investigates the effect of syringe infiltration, a commonly employed technique in plants, using a typical infiltration buffer solution. Noninvasive and real-time monitoring methods, including high-resolution thermal imaging and a porometer/fluorometer, were used to study the physiological responses and stress levels of the infiltrated plants. Our results revealed localized cell damage at the infiltration site due to syringe compression, but the overall cell viability and tissue integrity were largely unaffected. Thermography showed a temporary temperature increase of the leaves and stomatal conductance alterations postinfiltration, with leaf recovery in 3-6 days. Additionally, fluorescence measurements indicated a 6% decrease in maximum quantum efficiency (F v/F m) and a 34% decrease in photosystem II (ΦPSII) quantum yield, persisting for 5 days after infiltration, suggesting sustained photosystem efficiency changes. Our work highlights the need to consider the effect of infiltration when performing biological studies and aims to facilitate the optimization of protocols commonly used in plant science and biotechnology.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Deguchi M.; Bogush D.; Weeden H.; Spuhler Z.; Potlakayala S.; Kondo T.; Zhang Z. J.; Rudrabhatla S. Establishment and Optimization of a Hemp (Cannabis Sativa L.) Agroinfiltration System for Gene Expression and Silencing Studies. Sci. Rep. 2020, 10 (1), 3504.10.1038/s41598-020-60323-9. - DOI - PMC - PubMed
-
- Duc Tien N. Q. Transient Expression of Chi42 Genes from Trichoderma Asperellum in Nicotiana Benthamiana by Agroinfiltration. Int. J. Agric. Biol. 2021, 26 (01), 177–184. 10.17957/IJAB/15.1822. - DOI
-
- Vaghchhipawala Z.; Rojas C. M.; Senthil-Kumar M.; Mysore K. S. Agroinoculation and Agroinfiltration: Simple Tools for Complex Gene Function Analyses. In: Pereira A. (eds) Plant Reverse Genetics. Methods in Molecular Biology; Humana Press: Totowa, NJ, 2011, 678, 65–76. - PubMed
LinkOut - more resources
Full Text Sources
