Exploring the Potential Imaging Biomarkers for Parkinson's Disease Using Machine Learning Approach
- PMID: 39851285
- PMCID: PMC11762086
- DOI: 10.3390/bioengineering12010011
Exploring the Potential Imaging Biomarkers for Parkinson's Disease Using Machine Learning Approach
Abstract
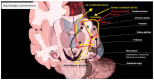
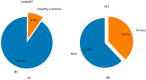
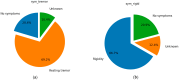
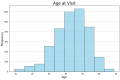
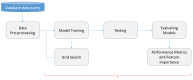
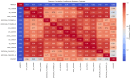
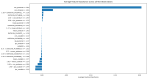
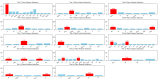
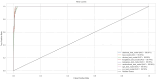
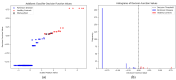
Parkinson's disease (PD) is a neurodegenerative disorder characterized by motor and neuropsychiatric symptoms resulting from the loss of dopamine-producing neurons in the substantia nigra pars compacta (SNc). Dopamine transporter scan (DATSCAN), based on single-photon emission computed tomography (SPECT), is commonly used to evaluate the loss of dopaminergic neurons in the striatum. This study aims to identify a biomarker from DATSCAN images and develop a machine learning (ML) algorithm for PD diagnosis. Using 13 DATSCAN-derived parameters and patient handedness from 1309 individuals in the Parkinson's Progression Markers Initiative (PPMI) database, we trained an AdaBoost classifier, achieving an accuracy of 98.88% and an area under the receiver operating characteristic (ROC) curve of 99.81%. To ensure interpretability, we applied the local interpretable model-agnostic explainer (LIME), identifying contralateral putamen SBR as the most predictive feature for distinguishing PD from healthy controls. By focusing on a single biomarker, our approach simplifies PD diagnosis, integrates seamlessly into clinical workflows, and provides interpretable, actionable insights. Although DATSCAN has limitations in detecting early-stage PD, our study demonstrates the potential of ML to enhance diagnostic precision, contributing to improved clinical decision-making and patient outcomes.
Keywords: AdaBoost; DATSCAN; Parkinson’s disease; basal ganglia; classification; machine learning.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Kozyolkin O., Revenko A., Medvedkova S. Parkinson’s Disease: Current Aspects of Diagnosis and Treatment. Zaporizhzhia State Medical University; Zaporizhzhia, Ukraine: 2017.
-
- Symptoms|Parkinson’s Disease. [(accessed on 25 September 2024)]. Available online: https://www.michaeljfox.org/symptoms.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

