Role of Virtual iMRI in Glioblastoma Surgery: Advantages, Limitations, and Correlation with iCT and Brain Shift
- PMID: 39851403
- PMCID: PMC11763980
- DOI: 10.3390/brainsci15010035
Role of Virtual iMRI in Glioblastoma Surgery: Advantages, Limitations, and Correlation with iCT and Brain Shift
Abstract
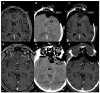
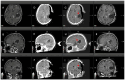
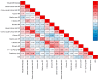
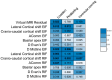
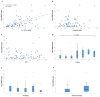
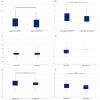
Background: Elastic image fusion (EIF) using an intraoperative CT (iCT) scan may enhance neuronavigation accuracy and compensate for brain shift. Objective: To evaluate the safety and reliability of the EIF algorithm (Virtual iMRI Cranial 4.5, Brainlab AG, Munich Germany, for the identification of residual tumour in glioblastoma surgery. Moreover, the impact of brain shift on software reliability is assessed. Methods: This ambispective study included 80 patients with a diagnosis of glioblastoma. Pre-operative MRI was elastically fused with an intraoperative CT scan (BodyTom; Samsung-Neurologica, Danvers, MA, USA) acquired at the end of the resection. Diagnostic specificity and the sensitivity of each tool was determined. The impact of brain shift on residual tumour was statistically analysed. An analysis of accuracy was performed through Target Registration Error (TRE) measurement after rigid image fusion (RIF) and EIF. A qualitative evaluation of each Virtual MRI image (VMRI) was performed. Results: VMRI identified residual tumour in 26/80 patients (32.5%), confirmed by post-operative MRI (true positive). Of these, 5 cases were left intentionally due to DES-positive responses, 8 cases underwent near maximal or subtotal resection, and 13 cases were not detected by iCT. However, in the other 27/80 cases (33.8%), VMRI reported residual tumour that was present neither on iCT nor on post-operative MRI (false positive). i-CT showed a sensitivity of 56% and specificity of 100%; VMRI demonstrated a sensitivity of 100% and specificity of 50%. Spearman correlation analysis showed a moderate correlation between pre-operative volume and VMRI tumour residual. Moreover, tumour involving insula or infiltrating more than one lobe displayed higher median values (p = 0.023) of virtual residual tumour. A statistically significant reduction towards lower TRE values after EIF was observed for test structures. Conclusions: Virtual iMRI was proven to be a feasible option to detect residual tumour. Its integration within a multimodal imaging protocol may provide neurosurgeons with intraoperatively updated imaging.
Keywords: Virtual iMRI; brain shift; brain tumour surgery; elastic image fusion; glioblastoma; intraoperative CT; rigid image fusion.
Conflict of interest statement
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Figures
References
LinkOut - more resources
Full Text Sources

