Extracellular Vesicles Derived from Human Umbilical Mesenchymal Stem Cells Transfected with miR-7704 Improved Damaged Cartilage and Reduced Matrix Metallopeptidase 13
- PMID: 39851510
- PMCID: PMC11763736
- DOI: 10.3390/cells14020082
Extracellular Vesicles Derived from Human Umbilical Mesenchymal Stem Cells Transfected with miR-7704 Improved Damaged Cartilage and Reduced Matrix Metallopeptidase 13
Abstract
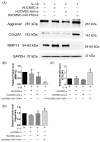
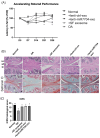
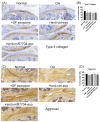
We aimed to explore the therapeutic efficacy of miR-7704-modified extracellular vesicles (EVs) derived from human umbilical cord mesenchymal stem cells (HUCMSCs) for osteoarthritis (OA) treatment. In vitro experiments demonstrated the successful transfection of miR-7704 into HUCMSCs and the isolation of EVs from these cells. In vivo experiments used an OA mouse model to assess the effects of the injection of miR-7704-modified EVs intra-articularly. Walking capacity (rotarod test), cartilage morphology, histological scores, and the expression of type II collagen, aggrecan, interleukin-1 beta, and matrix metalloproteinase 13 (MMP13) in the cartilage were evaluated. The EVs were characterized to confirm their suitability for therapeutic use. IL-1beta-treated chondrocytes increased type II collagen and decreased MMP13 after treatment with miR-7704-overexpressed EVs. In vivo experiments revealed that an intra-articular injection of miR-7704-overexpressed EVs significantly improved walking capacity, preserved cartilage morphology, and resulted in higher histological scores compared to in the controls. Furthermore, the decreased expression of MMP13 in the cartilage post treatment suggests a potential mechanism for the observed therapeutic effects. Therefore, miR-7704-overexpressed EVs derived from HUCMSCs showed potential as an innovative therapeutic strategy for treating OA. Further investigations should focus on optimizing dosage, understanding mechanisms, ensuring safety and efficacy, developing advanced delivery systems, and conducting early-phase clinical trials to establish the therapeutic potential of HUCMSC-derived EVs for OA management.
Keywords: MMP13; extracellular vesicles; human umbilical cord mesenchymal stem cells; miRNA; osteoarthritis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

