Blockade of TIPE2-Mediated Ferroptosis of Myeloid-Derived Suppressor Cells Achieves the Full Potential of Combinatory Ferroptosis and Anti-PD-L1 Cancer Immunotherapy
- PMID: 39851538
- PMCID: PMC11763990
- DOI: 10.3390/cells14020108
Blockade of TIPE2-Mediated Ferroptosis of Myeloid-Derived Suppressor Cells Achieves the Full Potential of Combinatory Ferroptosis and Anti-PD-L1 Cancer Immunotherapy
Abstract
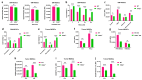
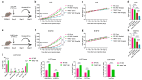
Although immune checkpoint blockade (ICB) therapy has attained unprecedented clinical success, the tolerance and immune suppression mechanisms evolved by tumor cells and their tumor microenvironment (TME) hinder its maximum anti-cancer potential. Ferroptosis therapy can partially improve the efficacy of ICB, but it is still subject to immune suppression by myeloid-derived suppressor cells (MDSCs) in the TME. Recent research suggests that an MDSC blockade can unleash the full therapeutic potential of the combined therapy of ferroptosis and ICB in liver cancer treatment. However, whether blocking the intrinsic ferroptosis pathways of MDSCs can relieve imidazole ketone erastin (IKE)-initiated ferroptosis-induced immune suppression and ultimately trigger the optimal therapeutic effect of the combined ferroptosis and ICB therapy is still unknown. Here, we report that TIPE2, a phospholipid transfer protein, regulated the ferroptosis susceptibility in MDSCs through reprogramming lipid peroxidation-related phosphatidylethanolamine (PE) and phosphatidylcholine (PC) species composition. TIPE2-deficient MDSCs resisted IKE-induced ferroptosis by up-regulating SLC7A11 and GPX4, and dissolved ferroptosis-induced immunosuppressive function by down-regulating lipid ROS whilst encouraging T cell proliferation and infiltration into tumor tissues to improve ferroptosis therapy. More importantly, TIPE2-deficient MDSCs achieved the full anti-tumor therapeutic potential of IKE-induced ferroptosis therapy and a PD-L1 blockade. These findings indicate that TIPE2 confers the ferroptosis sensitivity of MDSCs, and combining the targeting of the TIPE2 of MDSCs, ferroptosis therapy, and ICB is a novel therapeutic option for cancer treatment.
Keywords: MDSCs; TIPE2; cancer treatment; ferroptosis; immune checkpoint blockade.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Sharma P., Siddiqui B.A., Anandhan S., Yadav S.S., Subudhi S.K., Gao J., Goswami S., Allison J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021;11:838–857. doi: 10.1158/2159-8290.CD-20-1680. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

