Mebendazole Exerts Anticancer Activity in Ovarian Cancer Cell Lines via Novel Girdin-Mediated AKT/IKKα/β/NF-κB Signaling Axis
- PMID: 39851541
- PMCID: PMC11763501
- DOI: 10.3390/cells14020113
Mebendazole Exerts Anticancer Activity in Ovarian Cancer Cell Lines via Novel Girdin-Mediated AKT/IKKα/β/NF-κB Signaling Axis
Abstract
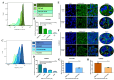
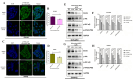
Mebendazole (MBZ), a benzimidazole anthelmintic and cytoskeleton-disrupting compound, exhibits antitumor properties; however, its action on ovarian cancer (OC) is not clearly understood. This study evaluates the effect of MBZ on OC cell lines OVCAR3 and OAW42, focusing on cell proliferation, migration, invasion, and cancer stemness. The underlying mechanisms, including cytoskeletal disruption, epithelial-mesenchymal transition (EMT), and signaling pathways, were explored. MBZ inhibited OVCAR3 and OAW42 cell proliferation in a dose- and time-dependent manner. Additionally, MBZ significantly impedes migration, spheroid invasion, colony formation, and stemness. In addition, it reduced actin polymerization and down-regulated CSC markers (e.g., CD24, CD44, EpCAM). Moreover, MBZ suppressed MMP-9 activity and inhibited the EMT marker as judged by decreased N-Cadherin and Vimentin and increased E-Cadherin. Furthermore, MBZ induced G2/M cell cycle arrest by modulating Cyclin B1, CDC25C, and WEE1. Also, it triggered apoptosis by disrupting mitochondrial membrane potential. Mechanistic studies revealed a significant downregulation of Girdin, an Akt modulator, along with reduced p-Akt, p-IKKα/β, and p-NF-κB, indicating MBZ's novel mechanism of action through the Girdin-mediated Akt/IKKα/β/NF-κB signaling axis. Thus, by targeting Girdin, MBZ presents a promising repurposed therapeutic strategy to inhibit cancer cell proliferation and metastasis in ovarian cancer.
Keywords: EMT; MMP-9; girdin; mebendazole; metastasis; ovarian cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Roett M.A., Evans P. Ovarian cancer: An overview. Am. Fam. Physician. 2009;80:609–616. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

