Neuroprotective Efficacy of Astragalus mongholicus in Ischemic Stroke: Antioxidant and Anti-Inflammatory Mechanisms
- PMID: 39851546
- PMCID: PMC11764225
- DOI: 10.3390/cells14020117
Neuroprotective Efficacy of Astragalus mongholicus in Ischemic Stroke: Antioxidant and Anti-Inflammatory Mechanisms
Abstract
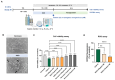
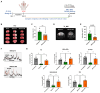
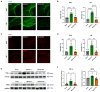
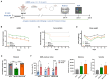
Stroke affects over 12 million people annually, leading to high mortality, long-term disability, and substantial healthcare costs. Although East Asian herbal medicines are widely used for stroke treatment, the pathways of operation they use remain poorly understood. Our study investigates the neuroprotective properties of Astragalus mongholicus (AM) in acute ischemic stroke using photothrombotic (PTB) and transient middle cerebral artery occlusion (tMCAO) mouse models, as well as an in vitro oxygen-glucose deprivation (OGD) model. Post-OGD treatment with AM improved cell viability in mouse neuroblastoma cells, likely by reducing reactive oxygen species (ROS). Mice received short-term (0-2 days) or long-term (0-27 days) AM treatment post-stroke. Infarct size was assessed using a 2,3,5-triphenyl tetrazolium chloride (TTC) staining procedure alongside magnetic resonance imaging (MRI). Neuroprotective metabolites including inositol (Ins), glycerophosphocholine+phosphocholine (GPc+ PCh), N-acetylaspartate+N-acetylaspartylglutamate (NAA+NAAG), creatine + phosphocreatine (Cr+PCr), and glutamine+glutamate (Glx) were analyzed via magnetic resonance spectroscopy (MRS). Gliosis was assessed using GFAP and Iba-1 immunohistochemical markers, while neurological deficits were quantified with modified neurological severity scores (mNSS). Motor and cognitive functions were assessed using cylinder, rotarod, and novel object recognition (NOR) tests. AM treatment significantly reduced ischemic damage and improved neurological outcomes in both acute and chronic stages of PTB and tMCAO models. Additionally, AM increased neuroprotective metabolites levels, reduced gliosis, and decreased oxidative stress, as evidenced by reduced inducible nitric oxide synthase (iNOS). These findings highlight the antioxidant properties of AM and its strong therapeutic potential for promoting recovery after ischemic stroke by alleviating neurological deficits, reducing gliosis, and mitigating oxidative stress.
Keywords: Astragalus mongholicus; brain injury; cognitive impairment; photothrombotic-induced mouse model; stroke; transient middle cerebral artery occlusion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

